Item AP059004: Use a model to explain how matter that flows into and out of a hibernating bear leads to the bear losing mass. (MC version)
Prior to winter, black bears eat large quantities of food and gain up to 30 pounds of additional body weight. The food that the bears eat is made up of carbon-containing molecules such as glucose, carbohydrates, fats, and proteins. When winter begins, the bears go into dens or caves to hibernate. After several months of hibernation, they wake up and leave their dens. Scientists who study hibernating bears have found that bears lose a quarter of their body weight during hibernation.

Observations of hibernating bears show that they breathe in and out during hibernation, but they do not eat, drink, or "go to the bathroom."
How is it possible for bears to stay alive during hibernation if they don’t eat anything?
Below is a model showing one of the chemical reactions taking place inside a bear that will help you understand how bears can stay alive during hibernation.
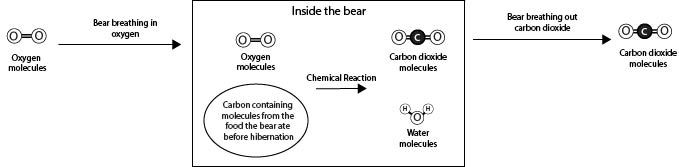
Using the model, answer the following questions about the processes happening inside the bear during hibernation.
1. How was the oxygen that the bear breathed in used by the bear to get the energy needed to stay alive?
A. The oxygen that the bear breathed in reacted with other molecules inside the bear in reactions that release energy and produce carbon dioxide molecules.
B. The oxygen that the bear breathed in reacted with other molecules inside the bear in reactions that release energy and produce water molecules.
C. The oxygen that the bear breathed in reacted with other molecules inside the bear in reactions that release energy and produce both carbon dioxide and water molecules.
D. The oxygen that the bear breathed in gave the bear energy. Oxygen was not involved in any of the chemical reactions.
2. Where did the carbon atoms that are in the carbon dioxide molecules that the bear breathed out come from?
A. The carbon-containing molecules from the food the bear ate.
B. The oxygen molecules that the bear breathed in.
C. The water molecules inside the bear’s body.
3. Using the model, how did the bear get the energy that it needed to stay alive during hibernation?
A. The bear got energy from carbon-containing molecules from food. No other molecules were involved in this process.
B. The bear got energy from oxygen molecules it breathes in. No other molecules were involved in this process.
C. The bear got energy from water molecules in the bear's body. No other molecules were involved in this process.
D. The bear got energy from reactions between carbon-containing molecules from food and oxygen molecules it breathed in.
E. The bear got energy from reactions between carbon-containing molecules from food and water molecules in the bear's body.

4. Now think about the fact that the bear lost weight during hibernation. Using the model, what caused the bear to decrease in mass during hibernation?
A. Some of the mass inside the bear was turned into energy during hibernation, causing the bear's mass to decrease.
B. The bear didn't eat during hibernation, and atoms from the food inside the bear were being destroyed, causing the bear's mass to decrease.
C. The bear breathed out more mass than it breathed in, causing the bear's mass to decrease while it hibernated.
D. The bear wasn't moving or exercising during hibernation, so the atoms that made up its muscles were being destroyed.
- Percent of Points Earned

- Points Earned
| Avg. Earned | Possible | Percent | |
|---|---|---|---|
| Q1 | 0.34 | 1 | 34% |
| Q2 | 0.41 | 1 | 41% |
| Q3 | 0.4 | 1 | 40% |
| Q4 | 0.09 | 1 | 9% |
- Overall Task Difficulty
| Total Points Earned | Total Points Possible | Total Percent | |
|---|---|---|---|
| 1.25 | 4 | 31% |
n = 233
Note: The total percent is a weighted average based on the total number of points earned divided by the total number of points possible.
- Science and Engineering Practices
- SEP6 Construct an explanation using models or representations.
- Crosscutting Concepts
- CC4 Models can be used to represent systems and their interactions--such as inputs, processes and outputs--and energy, matter, and information flows within systems.
CC5 Matter flows and cycles can be tracked in terms of the weight of the substances before and after a process occurs. The total weight of the substances does not change. This is what is meant by conservation of matter. Matter is transported into, out of, and within systems. - Disciplinary Core Ideas
- PS3.B The amount of energy transfer needed to change the temperature of a matter sample by a given amount depends on the nature of the matter, the size of the sample, and the environment.

