Item SC102002: The number of each kind of atom stays the same during a chemical reaction. (This item uses circles to represent atoms.)
The diagram below shows molecules before they react in a chemical reaction. Atoms are represented by circles, and molecules are represented by circles that are connected to each other. The different colored circles represent different kinds of atoms.

Which of the following diagrams could represent the molecules that result from the chemical reaction and why?
-

Because there were 6 atoms before the reaction and 6 atoms after the reaction. -
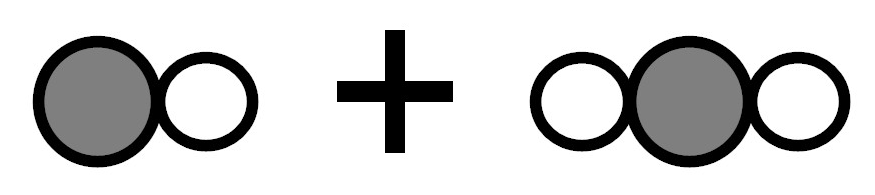
Because there were 2 kinds of molecules before the reaction and 2 kinds of molecules after the reaction. -
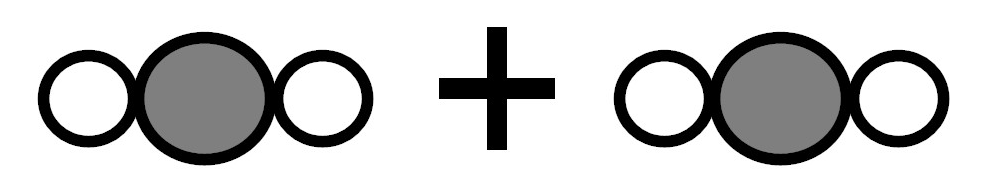
Because there were 4 white atoms and 2 gray atoms before the reaction and 4 white atoms and 2 gray atoms after the reaction. -
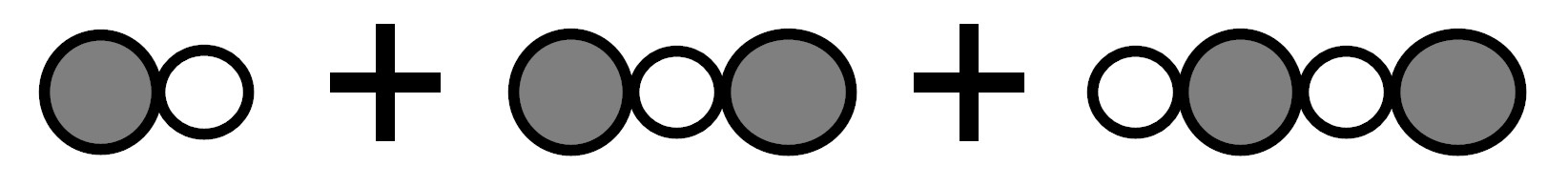
Because there were 3 molecules before the reaction and 3 molecules after the reaction.
- Distribution of Responses

- Students Responding Correctly
| Group | Correct | Total | Percent |
|---|---|---|---|
| Overall | 1483 | 2717 | 55% |
| Grades | |||
| 6–8 | 839 | 1603 | 52% |
| 9–12 | 630 | 1094 | 58% |
| Gender | |||
| Male | 694 | 1295 | 54% |
| Female | 753 | 1366 | 55% |
| Primary Language | |||
| English | 1319 | 2387 | 55% |
| Other | 123 | 261 | 47% |
- Disciplinary Core Ideas
- PS1.B Substances react chemically in characteristic ways. In a chemical process, the atoms that make up the original substances are regrouped into different molecules, and these new substances have different properties from those of the reactants.
PS1.B The total number of each type of atom is conserved, and thus the mass does not change.

