Item SC090003: The number of each kind of atom stays the same during the reaction between copper and oxygen. (This item uses circles to represent atoms.)
When heated, oxygen reacts with copper to form copper oxide.
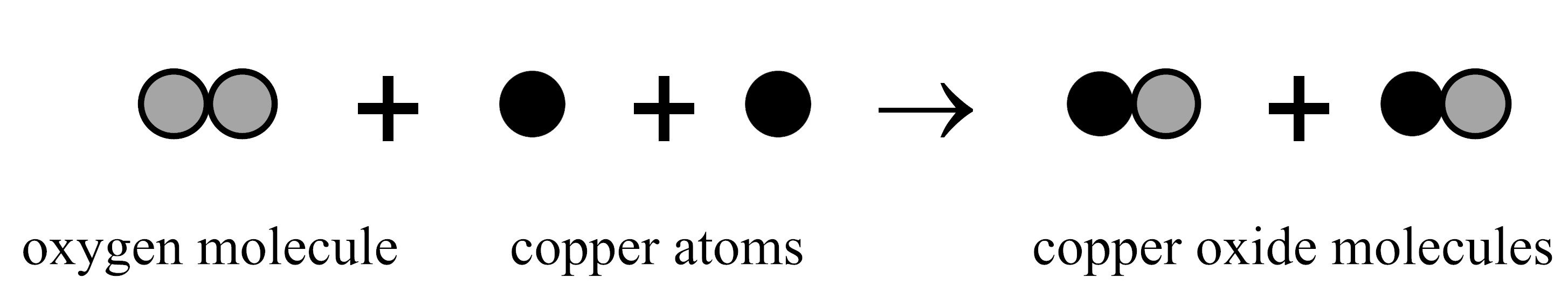
If this reaction occurs in a sealed container, will the mass of the container and everything in it increase, decrease, or stay the same and why?
- The mass will stay the same because the number of each kind of atom stays the same.
- The mass will decrease because two substances combine to form one substance.
- The mass will increase because a new kind of molecule is formed.
- More information is needed to tell if the mass will change.
- Distribution of Responses

- Students Responding Correctly
| Group | Correct | Total | Percent |
|---|---|---|---|
| Overall | 1820 | 3879 | 47% |
| Grades | |||
| 6–8 | 597 | 1611 | 37% |
| 9–12 | 1215 | 2251 | 54% |
| Gender | |||
| Male | 918 | 1914 | 48% |
| Female | 865 | 1887 | 46% |
| Primary Language | |||
| English | 1630 | 3395 | 48% |
| Other | 143 | 397 | 36% |
- Disciplinary Core Ideas
- PS1.B Substances react chemically in characteristic ways. In a chemical process, the atoms that make up the original substances are regrouped into different molecules, and these new substances have different properties from those of the reactants.
PS1.B The total number of each type of atom is conserved, and thus the mass does not change.

