Item AP062003: Use ideas about energy and chemical reactions to explain how a small flame from a candle can produce a large flame from a bubble filled with methane. (MC Version)
Natural gas is a fossil fuel that is found deep beneath the surface of the earth. It is made mostly of methane. When natural gas is burned, methane (CH4) reacts with oxygen (O2) to form carbon dioxide (CO2) and water (H2O) as represented below.
CH4 + 2 O2 → CO2 + 2 H2O
As methane reacts with oxygen, a flame is observed, and the temperature of the surroundings increases. The video below shows what happens when a soap bubble filled with methane gas reacts with oxygen in the air. The flame from a candle is used to start the reaction.
A student notices that the flame from the methane-oxygen reaction is much bigger than the flame from the candle. The student wonders how such a small flame from the candle could produce such a large flame from the bubble filled with methane. The student knows that a flame is an indicator that energy is being released during the chemical reaction and that all chemical reactions involve breaking and forming bonds between atoms.
1. To answer the question about why such a large flame was produced, the student starts to think about the energy changes that occur during the breaking and forming of bonds. Which of the following describes the energy changes associated with breaking and forming bonds during a chemical reaction?
A. Energy is given off as bonds are broken, and energy is given off when bonds are formed.
B. Energy is given off as bonds are broken, but energy is required to form bonds.
C. Energy is required to break bonds, but energy is given off as bonds are formed.
D. Energy is required to break bonds, and energy is required to form bonds.
Next, he decides to draw models to help him think about the energy associated with the atoms and molecules in the chemical reaction system. He knows that to form the products, bonds between the atoms of the reactants need to break and new bonds need to form. So, he decides to model a hypothetical transition state where all the bonds are broken and the atoms are separated.
2. Which bar graph best represents the energy associated with the methane and oxygen molecules and with the separated carbon, hydrogen, and oxygen atoms?
| A. |
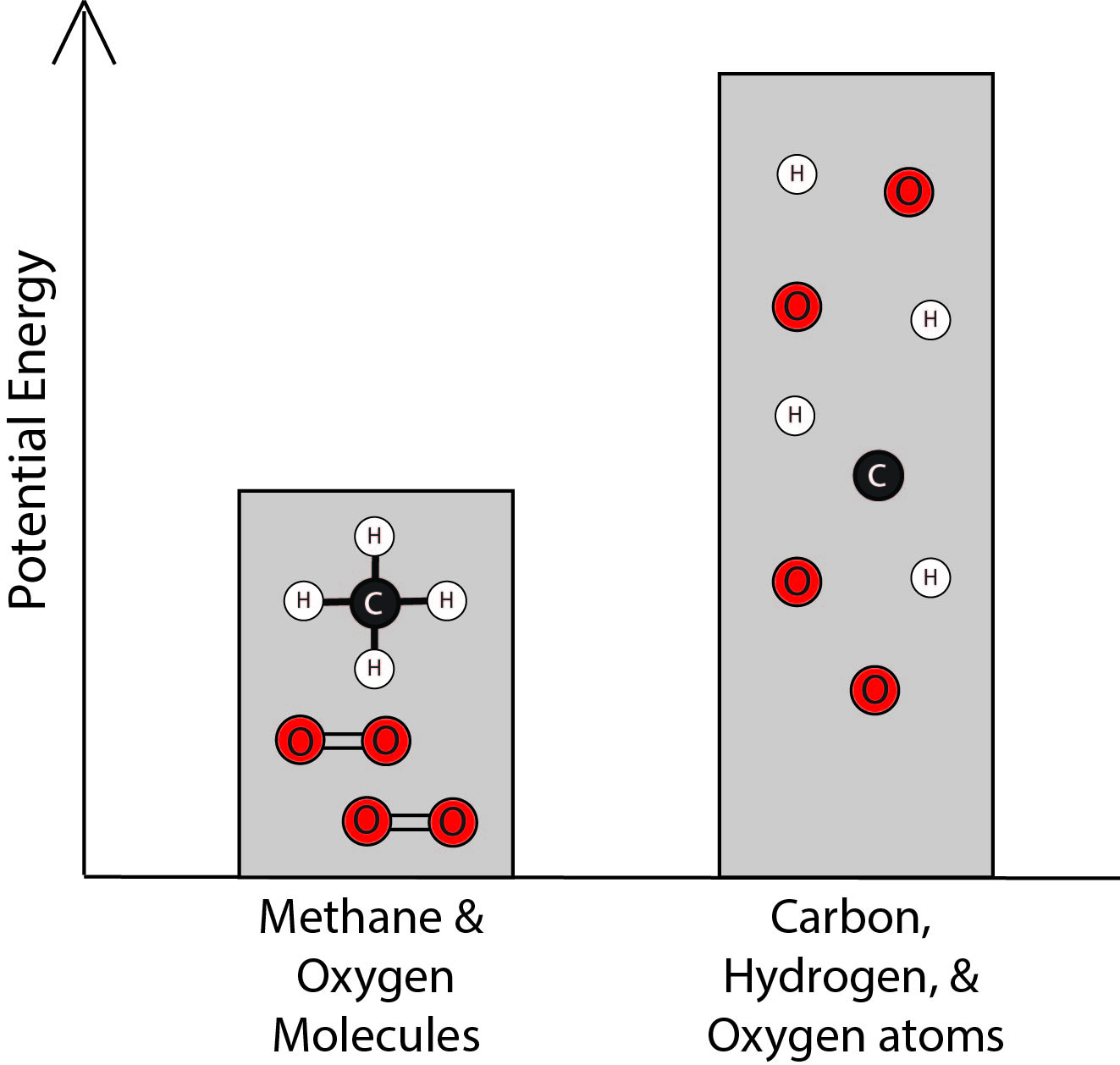
|
B. |
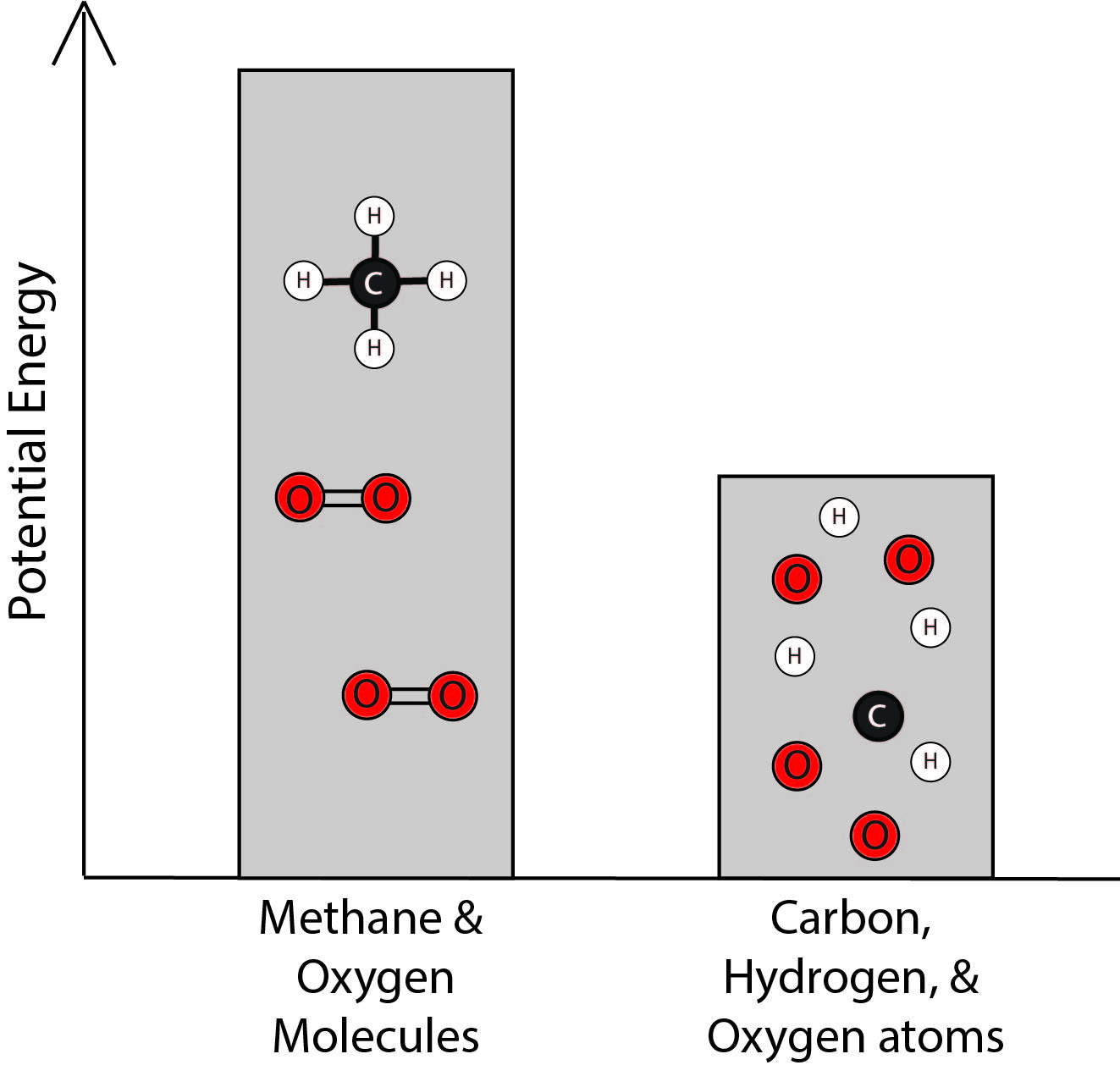
|
|
| C. |
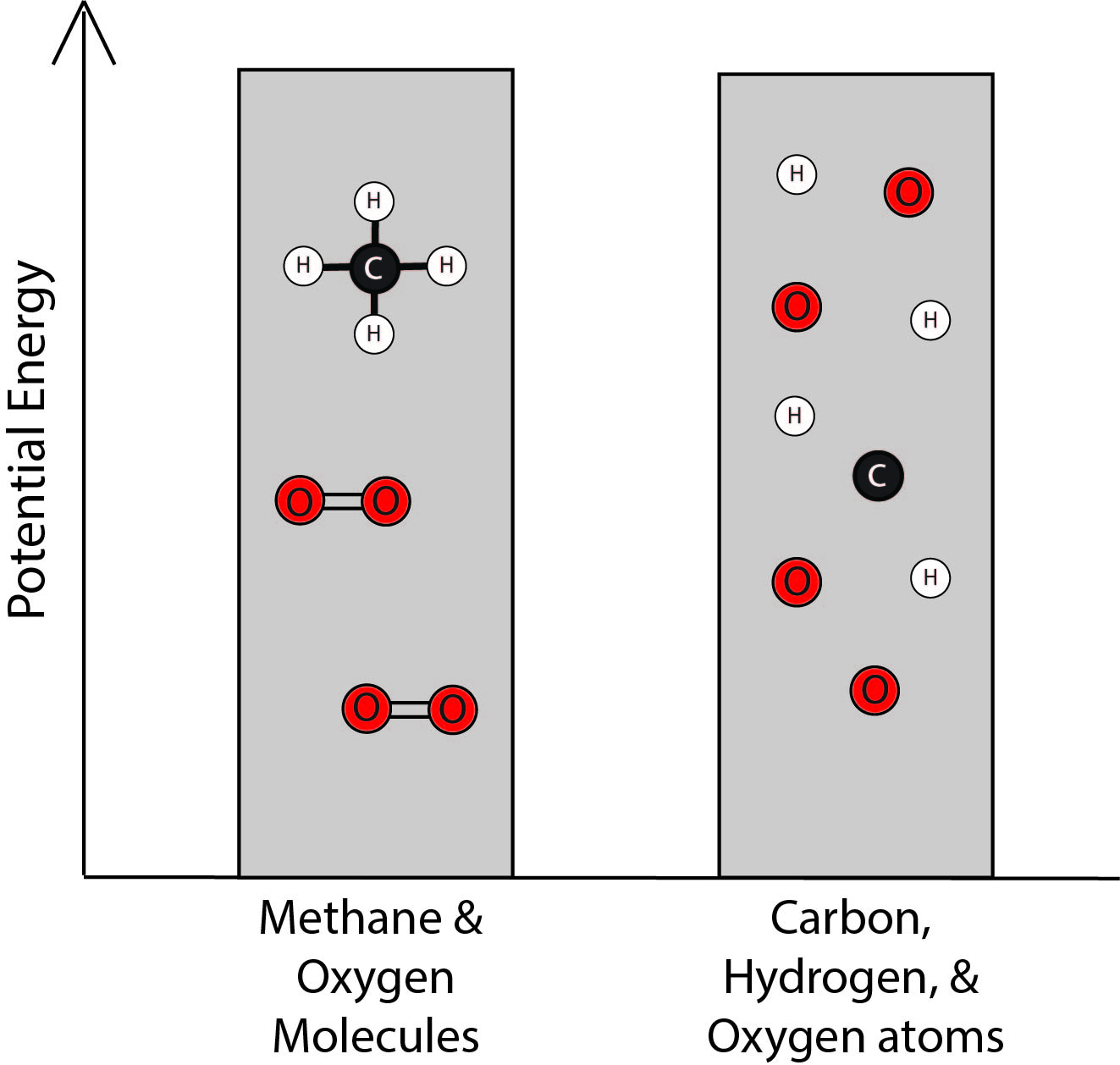
|
|||
3. Which bar graph best represents the energy associated with the separated carbon, hydrogen, and oxygen atoms and with the water and carbon dioxide molecules?
| A. |
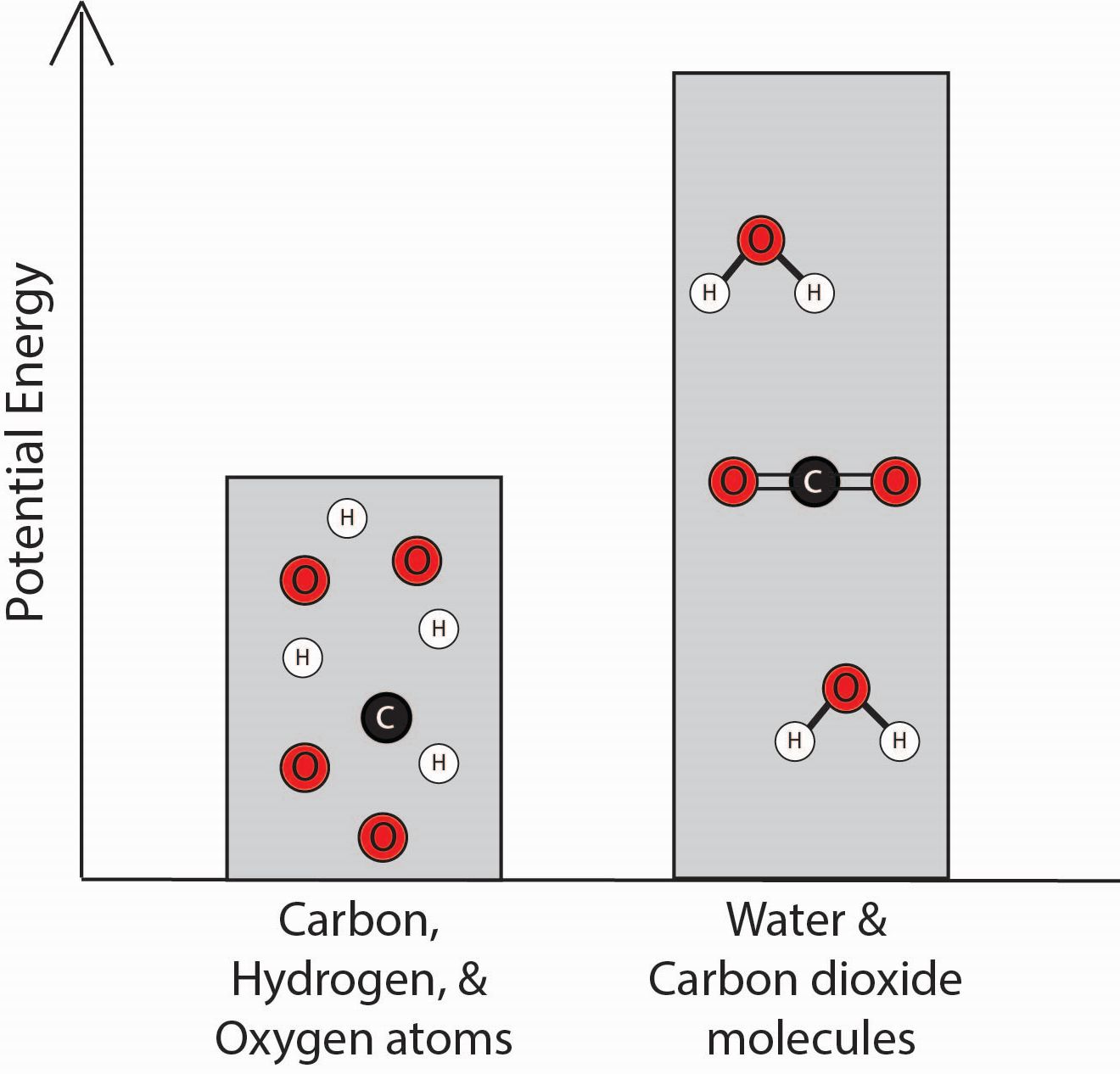
|
B. |
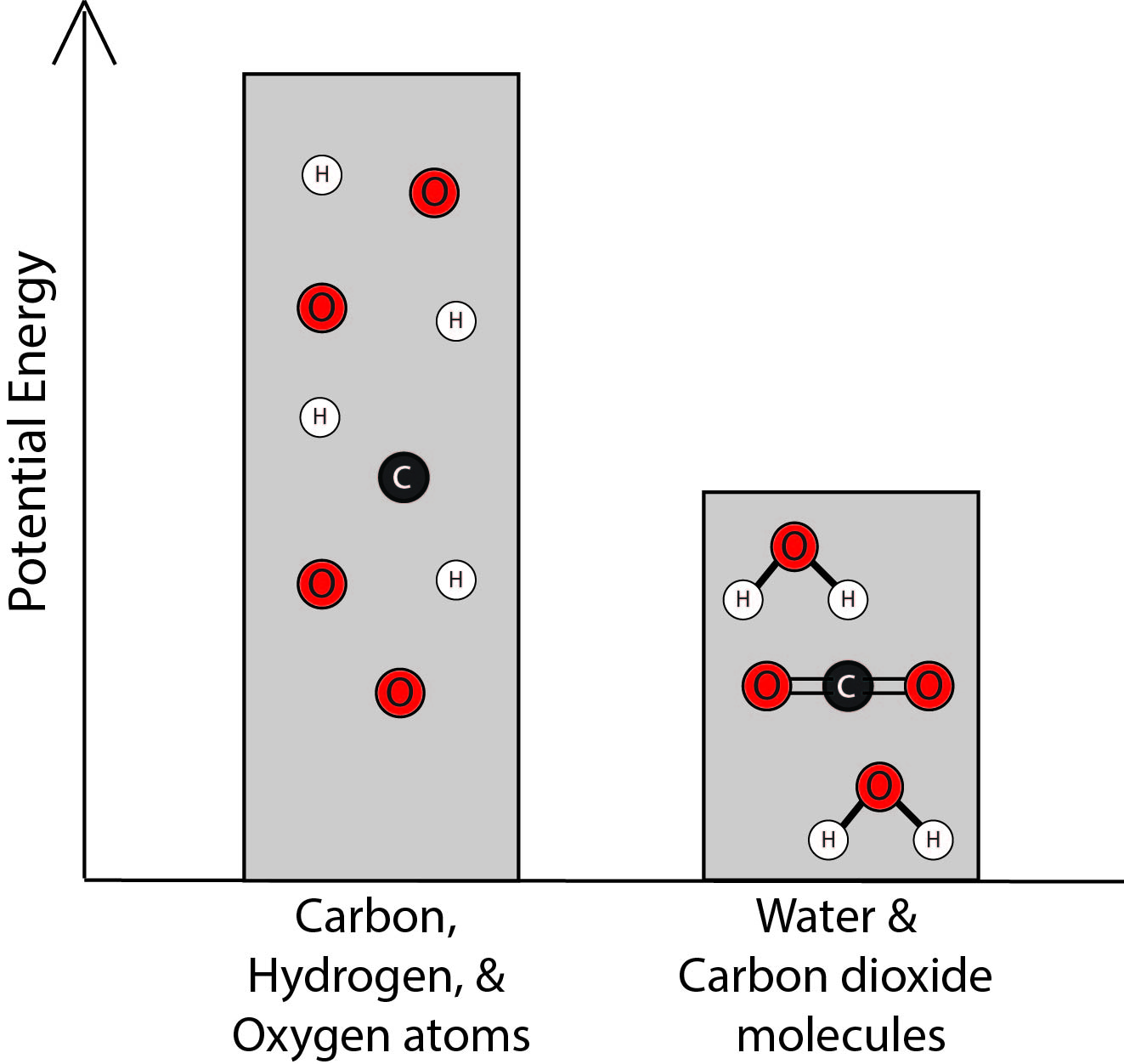
|
|
| C. |
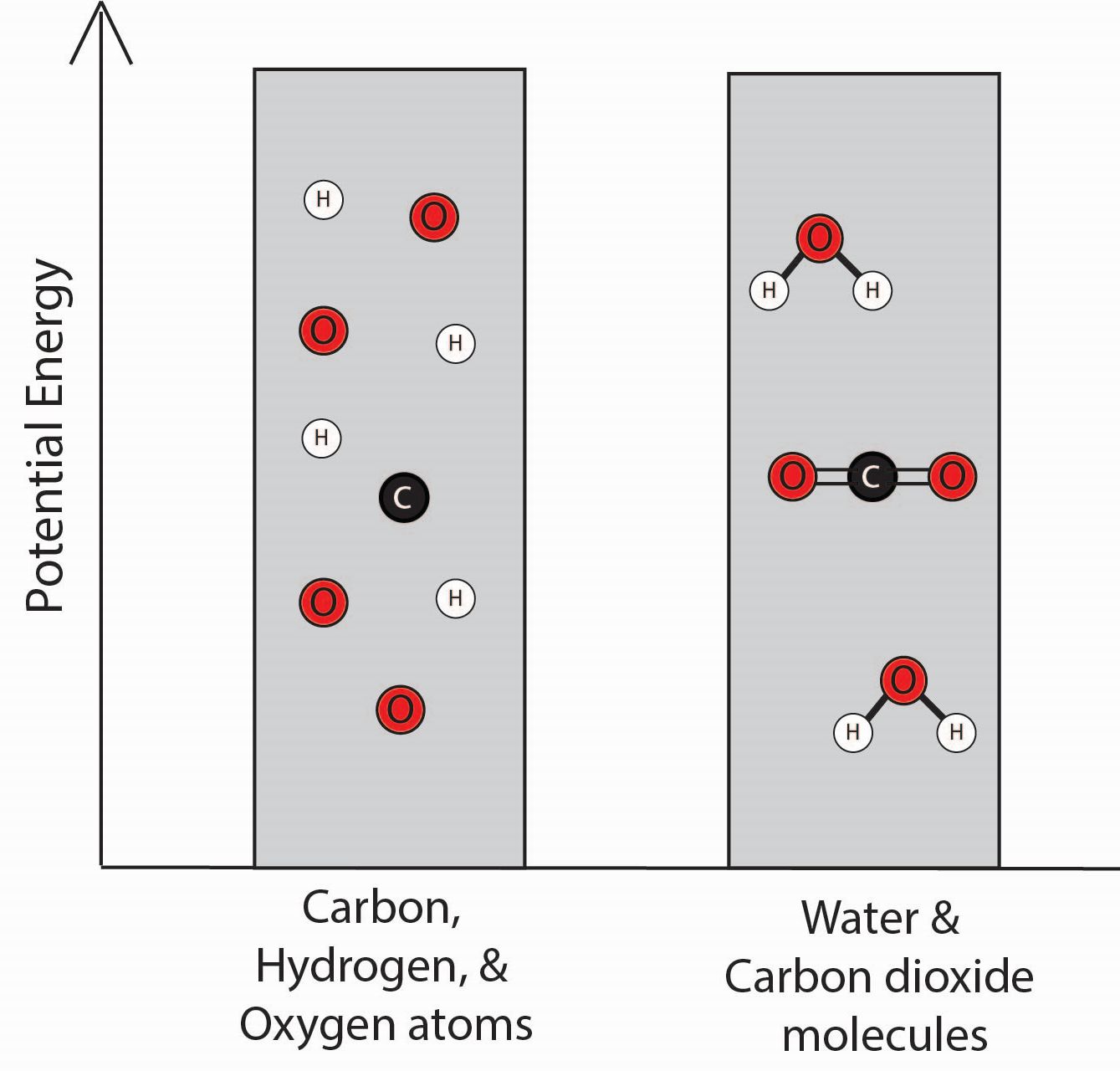
|
|||
The student knows that there are attractive forces between the atoms that make up the molecules in the chemical reaction system.
4. Using the idea that there are attractive forces between atoms, explain why the graphs you selected in Questions 2 and 3 best represent the energy associated with the different configurations of atoms. Be sure to link your explanation to the graphs you selected.
5. The student wants to use the graphs to make a simplified model that represents the energy changes that occur during the reaction. Which model represents the potential energy in the chemical reaction system for each configuration?
| A. |
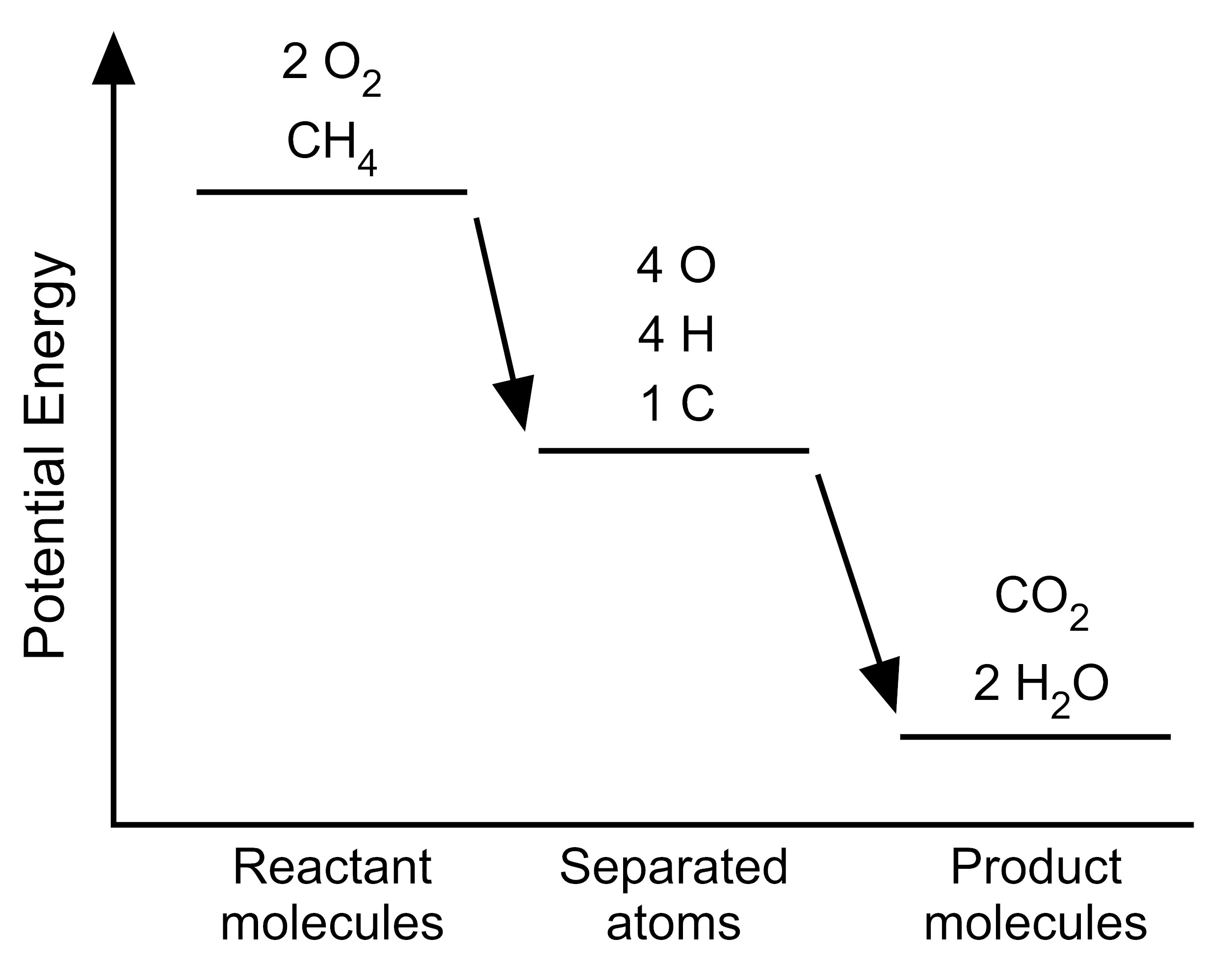
|
B. |
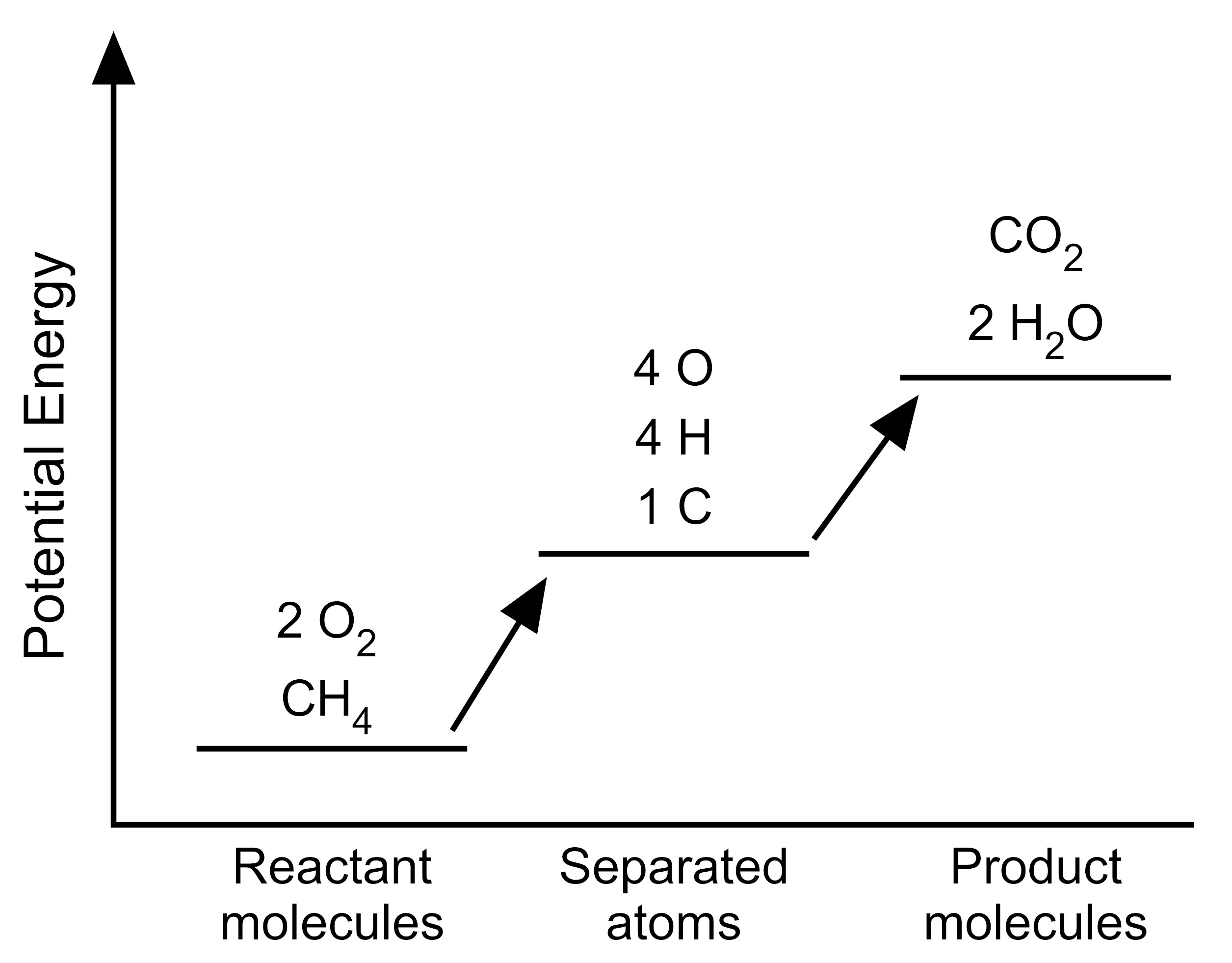
|
|
| C. |
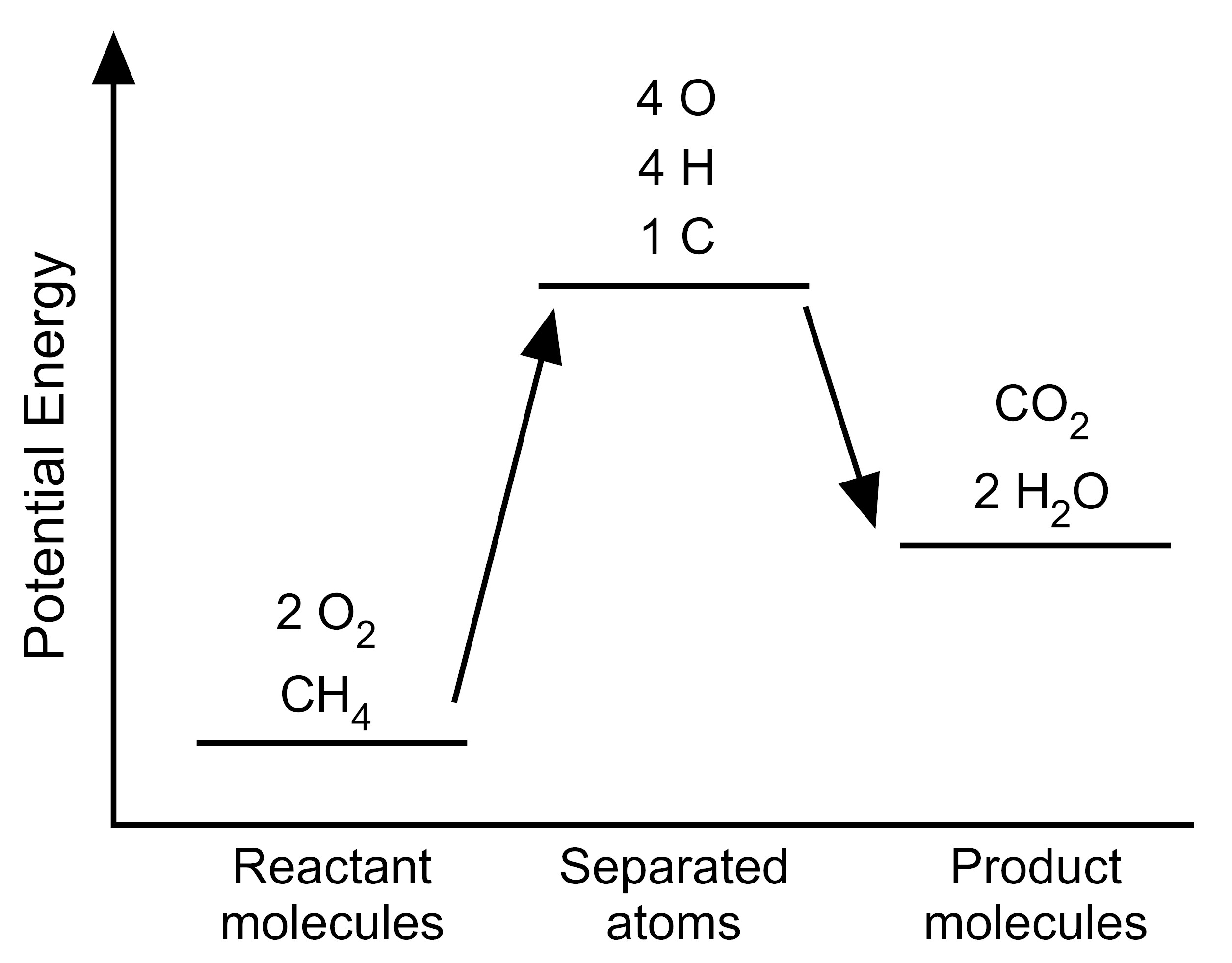
|
D. |
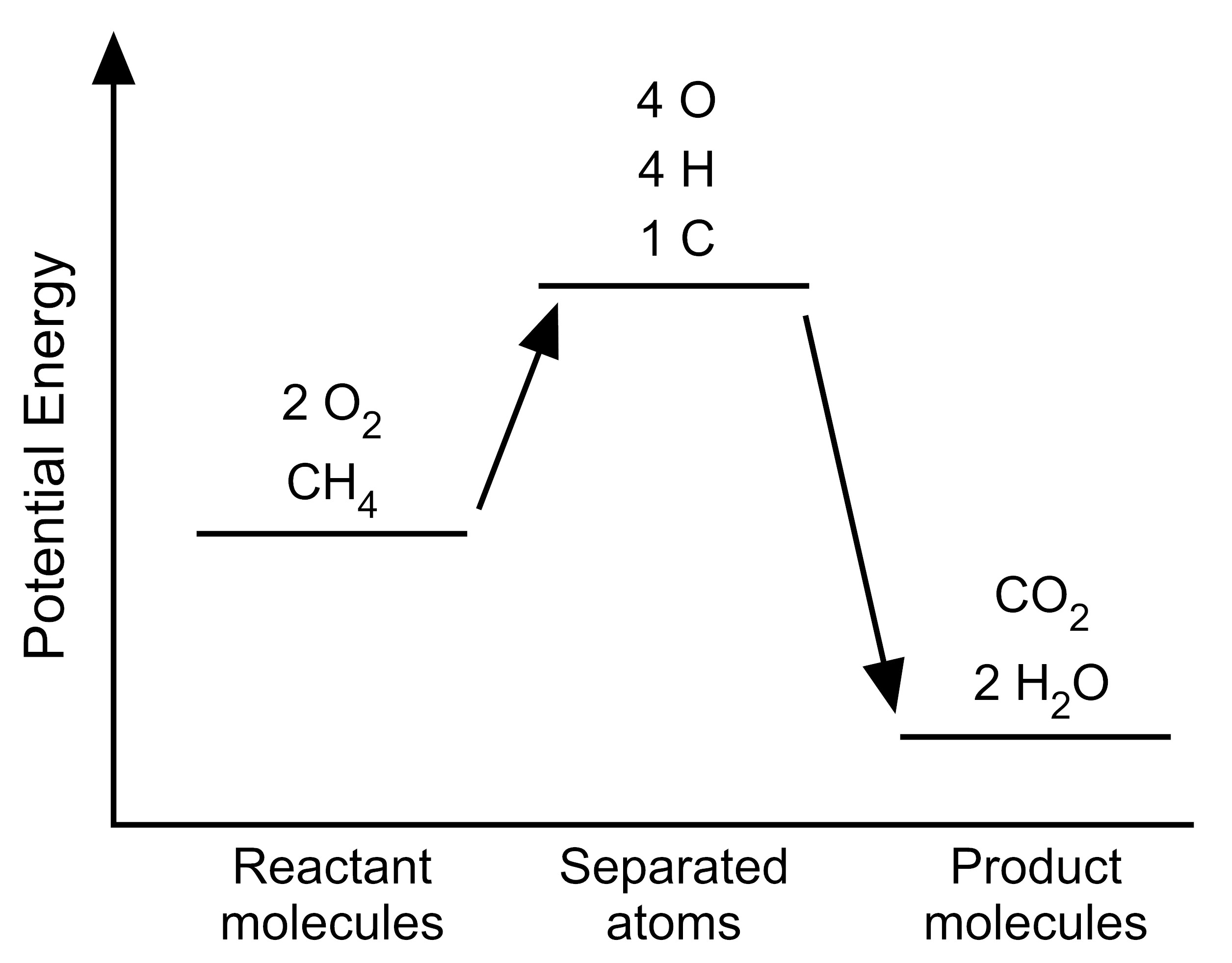
|
|
| E. |
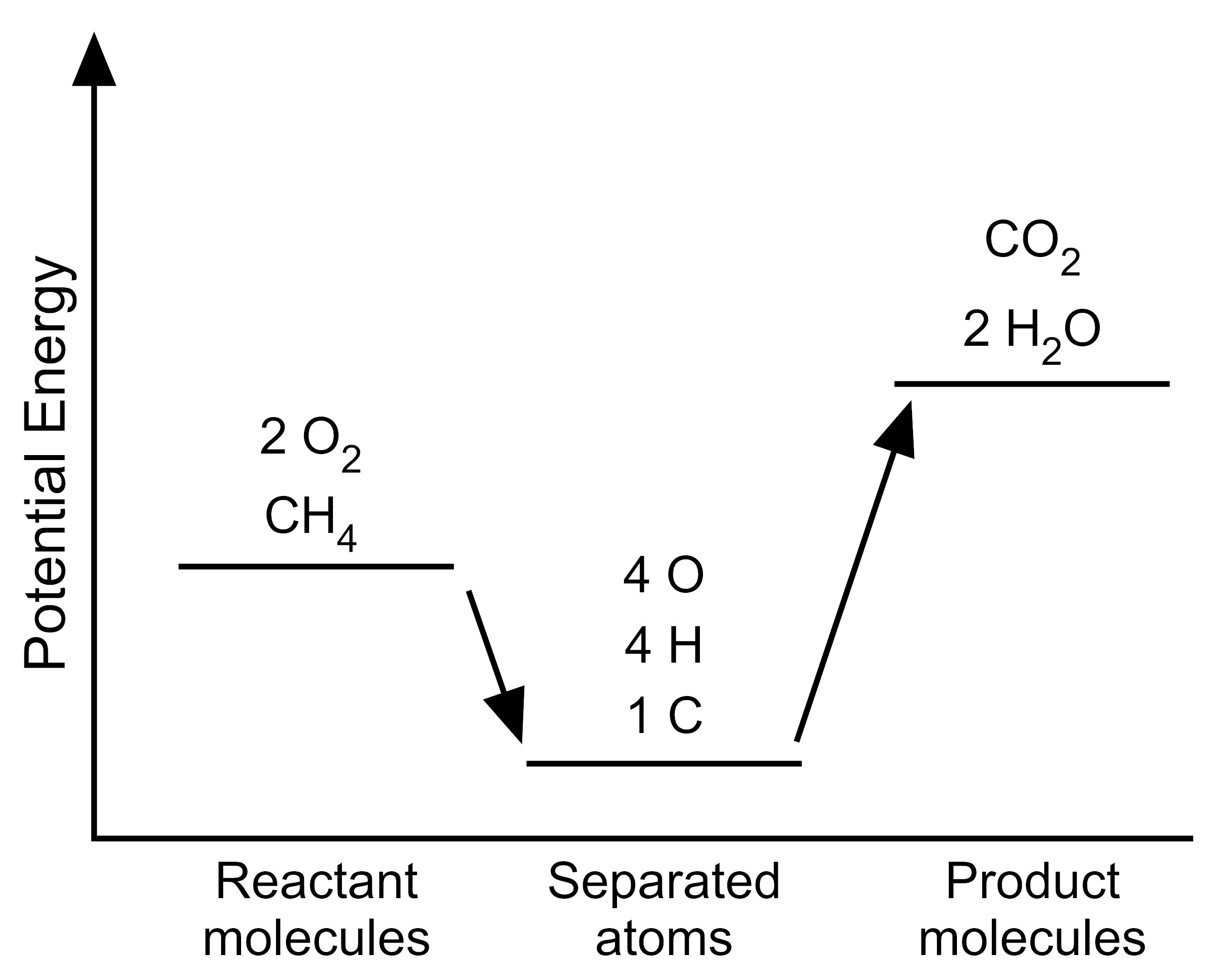
|
F. |
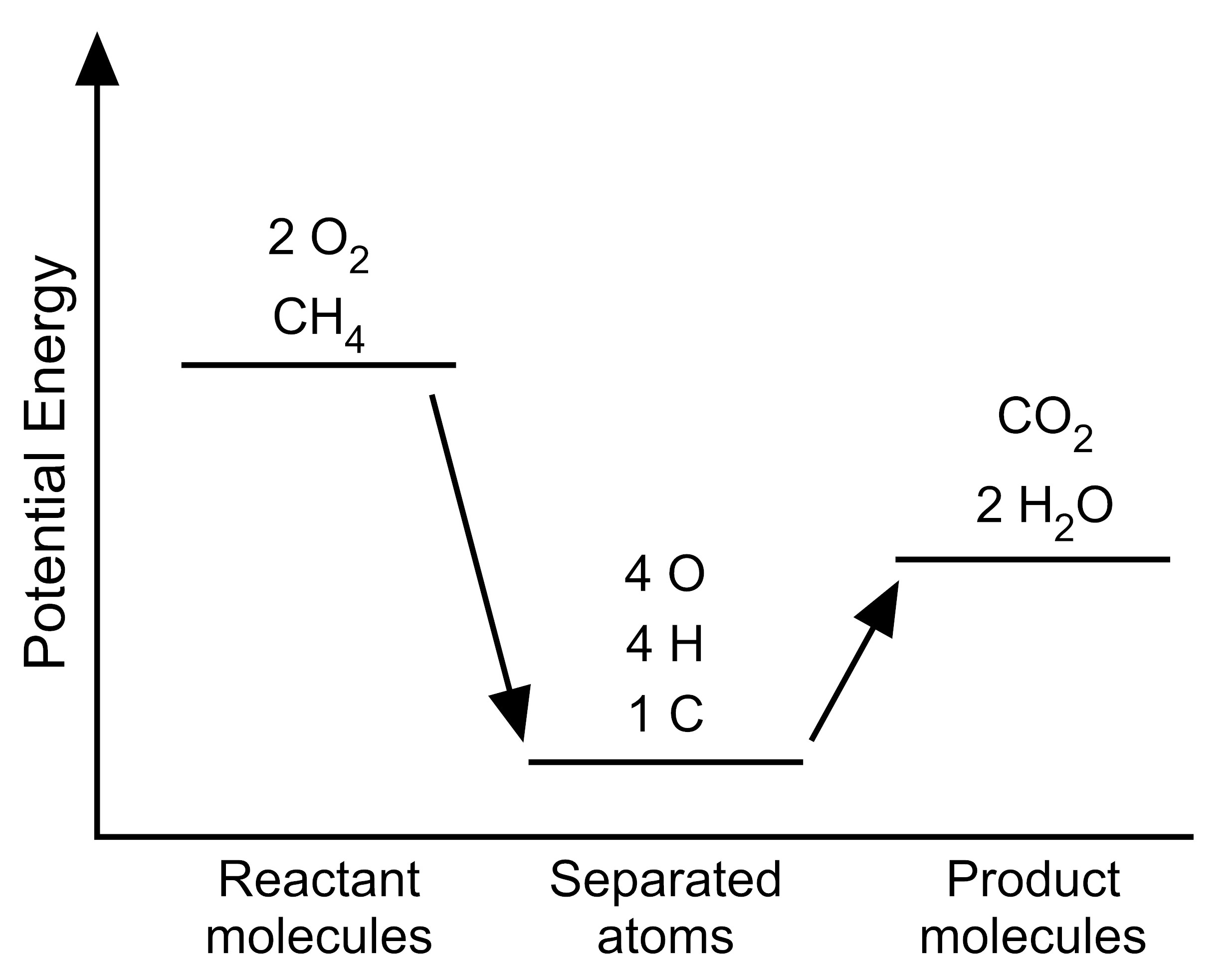
|
6. Why was a little bit of energy from the candle needed to start the methane-oxygen reaction even though a lot of energy was released during the reaction?
A. An input of energy was needed to start the reaction because bonds between the atoms of the product molecules need to be formed, and an initial input of energy is needed to form bonds.
B. An input of energy was needed to start the reaction because bonds between the atoms of the reactant molecules must be broken for a reaction to occur, and an initial input of energy is required to break bonds.
C. An input of energy was needed to start the reaction because energy is stored in the bonds between the atoms of the reactant molecules, and a small amount of energy is needed to trigger the release of that stored energy.
D. An input of energy was needed to start the reaction because chemical reactions can only occur at high temperatures, and the flame from the candle heated the methane up to a temperature high enough for the reaction to occur.
- Percent of Points Earned

- Points Earned
| Avg. Earned | Possible | Percent | |
|---|---|---|---|
| Q1 | 0.21 | 1 | 21% |
| Q2 | 0.32 | 1 | 32% |
| Q3 | 0.32 | 1 | 32% |
| Q4 | 0.03 | 2 | 2% |
| Q5 | 0.2 | 1 | 20% |
| Q6 | 0.25 | 1 | 25% |
- Overall Task Difficulty
| Total Points Earned | Total Points Possible | Total Percent | |
|---|---|---|---|
| 1.32 | 7 | 19% |
n = 193
Note: The total percent is a weighted average based on the total number of points earned divided by the total number of points possible.
- Science and Engineering Practices
- SEP2 Evaluate merits and limitations of two different models of the same proposed tool, process, mechanism or system in order to select or revise a model that best fits the evidence or design criteria.
SEP2 Develop and/or use multiple types of models to provide mechanistic accounts and/or predict phenomena, and move flexibly between model types based on merits and limitations.
SEP6 Apply scientific reasoning, theory, and/or models to link evidence to the claims to assess the extent to which the reasoning and data support the explanation or conclusion - Crosscutting Concepts
- CC2 Cause and effect relationships can be suggested and predicted for complex natural and human designed systems by examining what is known about smaller scale mechanisms within the system.
CC5 Changes of energy and matter in a system can be described in terms of energy and matter flows into, out of, and within that system. - Disciplinary Core Ideas
- PS1.A A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
PS1.B Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
PS2.B Attraction and repulsion between electric charges at the atomic scale explain the structure, properties, and transformations of matter, as well as the contact forces between material objects.
PS3.C When two objects interacting through a field change relative position, the energy stored in the field is changed.




