Item AP043006: Write an argument comparing different reactions in a combustion engine and use bond energy data and potential energy diagrams to explain which reaction releases more energy.
Internal combustion engines, which are used in many cars, transform potential energy into kinetic energy. A group of engineers is working with an engine in which butane reacts with oxygen. They find that different chemical reactions can occur depending on the ratio of butane to oxygen. These different reactions release different amounts of energy. They want to adjust the ratio of butane to oxygen to find the reaction that releases the largest amount of energy.
They start by adjusting the engine so that there is an abundance of oxygen (O2). When there is an abundance of oxygen, the butane (C4H10) reacts with the oxygen (O2) to produce carbon dioxide (CO2) and water (H2O) as shown by the chemical reaction below.
Chemical reaction with abundant oxygen
2C4H10 + 13O2 --> 8CO2 + 10H2O
To estimate how much energy will be released during this reaction, the engineers sum the bond energies associated with the reactant molecules and product molecules.
|
|
|
Sum of bond energies (kJ) |
|
Reactant molecules |
2C4H10 + 13O2 |
17,479 |
|
Product molecules |
8CO2 + 10H2O |
22,044 |
1. Based on the information in the table, how much energy is required to separate all of the atoms that make up the reactant molecules?
A. 0 kJ
B. 17,479 kJ
C. 22,044 kJ
D. 4,565 kJ (22,044 kJ - 17,479 kJ)
E. 39,523 kJ (22,044 kJ + 17,479 kJ)
2. Based on the information in the table, what is the estimated amount of energy released by the chemical reaction?
A. 0 kJ
B. 17,479 kJ
C. 22,044 kJ
D. 4,565 kJ (22,044 kJ - 17,479 kJ)
E. 39,523 kJ (22,044 kJ + 17,479 kJ)
The engineers create a potential energy diagram for the reactant and product molecules. The diagram shows the potential energies of: (1) the reactant molecules (2) the atoms if they were able to be completely separated from one another, and (3) the product molecules.
Potential energy diagram with abundant oxygen
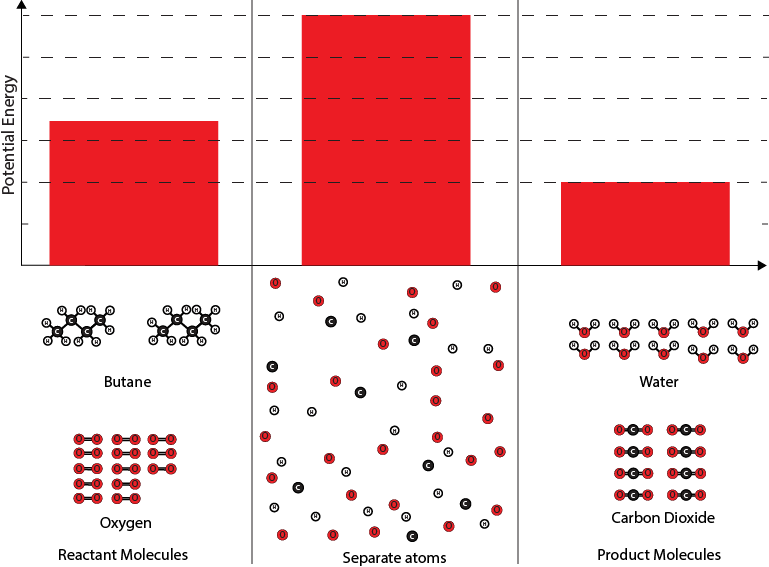
3. Given that there are attractive forces between atoms that make up the molecules involved in the chemical reaction, why is the potential energy of separated atoms greater than the potential energy of the reactant and product molecules?
4. How is the bond energy associated with the reactant molecules (17,479 kJ) represented in the potential energy diagram?
A. The bond energy is equal to the height of the bar for the reactant molecules.
B. The bond energy is equal to the height of the bar for the separated atoms.
C. The bond energy is equal to the difference in the heights of the bars for the reactant molecules and the separated atoms.
D. The bond energy is equal to the difference in the heights of the bars for the reactant molecules and the product molecules.
5. How is the net amount of energy released during the chemical reaction represented in the potential energy diagram?
A. The net amount of energy released is equal to the height of the bar for the product molecules.
B. The net amount of energy released is equal to the height of the bar for the separated atoms.
C. The net amount of energy released is equal to the difference in the heights of the bar for the product molecules and the separated atoms.
D. The net amount of energy released is equal to the difference in the heights of the bar for the reactant molecules and the product molecules.
Next, the engineers reduce the amount of oxygen available to the engine. They find that when the amount of oxygen is reduced butane reacts with oxygen to produce carbon dioxide, water, and carbon monoxide (CO). The chemical equation, total bond energies, and potential energy diagram for the chemical reaction in the reduced oxygen environment is shown below.
Chemical reaction with reduced oxygen
2C4H10 + 12O2 --> 6CO2 + 10H2O + 2CO
|
|
Total Bond Energy (kJ) |
|
| Reactant molecules |
2C4H10 + 12O2 |
16,984 |
| Product molecules |
6CO2 + 10H2O + 2CO |
20,992 |
Potential energy diagram with reduced oxygen
_energy_released.png)
6. How does the net amount of energy released during the chemical reaction in the reduced oxygen environment compare to the net amount of energy released during the chemical reaction when oxygen was abundant? Support your argument using data from the two tables of bond energies and the two potential energy diagrams.
- Percent of Points Earned

- Points Earned
| Avg. Earned | Possible | Percent | |
|---|---|---|---|
| Q1 | 0.31 | 1 | 31% |
| Q2 | 0.17 | 1 | 17% |
| Q3 | 0.1 | 2 | 5% |
| Q4 | 0.34 | 1 | 34% |
| Q5 | 0.28 | 1 | 28% |
| Q6 | 0.23 | 4 | 6% |
- Overall Task Difficulty
| Total Points Earned | Total Points Possible | Total Percent | |
|---|---|---|---|
| 1.44 | 10 | 14% |
n = 207
Note: The total percent is a weighted average based on the total number of points earned divided by the total number of points possible.
- Science and Engineering Practices
- SEP2 Develop, revise, and/or use a model based on evidence to illustrate and/or predict the relationships between systems or between components of a system.
SEP7 Construct, use, and/or present an oral and written argument or counter-arguments based on data and evidence. - Disciplinary Core Ideas
- PS1.A A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
PS1.B Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.
PS3.A These relationships are better understood at the microscopic scale, at which all of the different manifestations of energy can be modeled as a combination of energy associated with the motion of particles and energy associated with the configuration (relative position of the particles). In some cases the relative position energy can be thought of as stored in fields (which mediate interactions between particles). This last concept includes radiation, a phenomenon in which energy stored in fields moves across space.

