Item AP033006: Select a model that illustrates the energy changes when a hummingbird uses sucrose to get energy. Using the model, explain how energy flows into and out of the chemical reaction system.
A family decides to put a hummingbird feeder in their backyard to attract more hummingbirds. Hummingbirds, like many animals, can get energy by eating sugars such as common table sugar known as sucrose. So, they fill the hummingbird feeder with a mixture of sucrose and water.

Sucrose molecules inside the hummingbird will react with oxygen molecules that the hummingbird breathes in to form carbon dioxide molecules and water molecules. Your task is to explain the energy changes that occur when a hummingbird uses sucrose to get energy. First, think about the potential energy difference between the reactant molecules (sucrose and oxygen) and the product molecules (carbon dioxide and water).
1. In order for the hummingbird to get energy from this chemical reaction, how must the potential energy of the reactant molecules (sucrose and oxygen) compare to the potential energy of the product molecules (carbon dioxide and water)?
A. The reactant molecules must have more potential energy than the product molecules.
B. The reactant molecules must have less potential energy than the product molecules.
Next, you will make a diagram to illustrate the energy changes that take place during the chemical reaction. Below are several different diagrams. The bars in the diagrams represent the potential energy associated with the chemical reaction system at three times:
- The initial state before the oxygen and sucrose molecules have reacted,
- A hypothetical intermediate state* where all the atoms are separated, and
- The final state after the carbon dioxide and water molecules have formed.
(*During the reaction, atoms are separating and coming together to form new molecules. There is never a time when they are all completely separated at the same time. It is shown that way here to simplify the diagrams.)
2. Which of the diagrams best shows the potential energy changes of the chemical reaction system during the reaction between sucrose and oxygen molecules?
| A. |
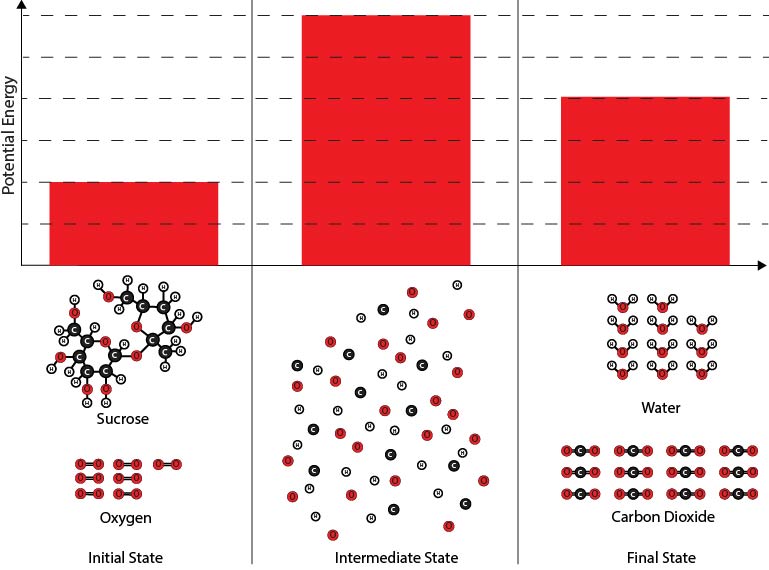
|
| B. |

|
| C. |
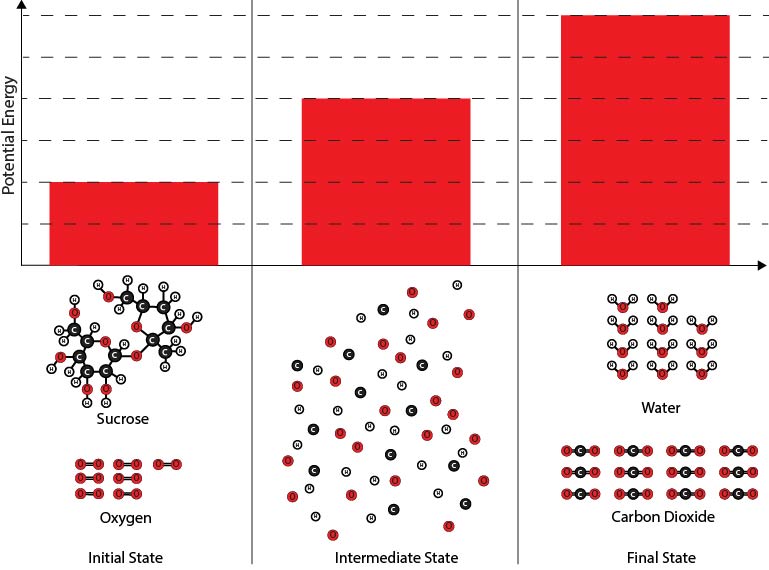
|
| D. |
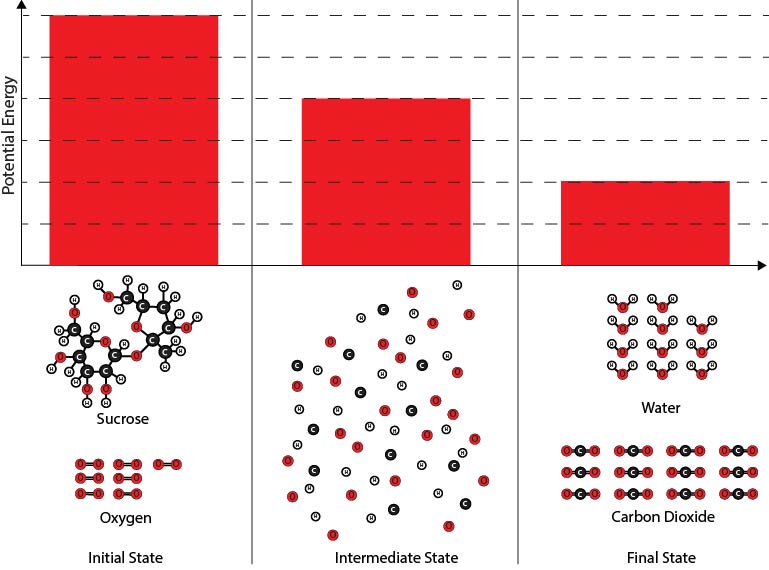
|
Now, use your diagram to help explain the energy changes that occur during the chemical reaction between sucrose and oxygen.
3. How does the amount of energy in the chemical reaction system change when going from the initial state to the intermediate state? In your explanation, be sure to write about energy inputs or outputs to the chemical reaction system.
4. How does the amount of energy in the chemical reaction system change when going from the intermediate state to the final state? In your explanation, be sure to write about energy inputs or outputs to the chemical reaction system.
5. Given that there are attractive forces between atoms that make up the molecules involved in the chemical reaction, explain why the energy of the chemical reaction system changes when the arrangement of atoms changes (for example, when going from molecules in the initial state to separated atoms in the intermediate state and going from the intermediate state to different molecules in the final state).
- Percent of Points Earned

- Points Earned
| Avg. Earned | Possible | Percent | |
|---|---|---|---|
| Q1 | 0.49 | 1 | 49% |
| Q2 | 0.2 | 1 | 20% |
| Q3 | 0.2 | 3 | 7% |
| Q4 | 0.3 | 3 | 10% |
| Q5 | 0.03 | 2 | 2% |
- Overall Task Difficulty
| Total Points Earned | Total Points Possible | Total Percent | |
|---|---|---|---|
| 1.22 | 10 | 12% |
n = 213
Note: The total percent is a weighted average based on the total number of points earned divided by the total number of points possible.
- Science and Engineering Practices
- SEP2 Develop and/or use multiple types of models to provide mechanistic accounts and/or predict phenomena, and move flexibly between model types based on merits and limitations.
SEP2 Use a model to predict the relationships between systems or between components of a system. - Crosscutting Concepts
- CC5 Changes of energy and matter in a system can be described in terms of energy and matter flows into, out of, and within that system.
- Disciplinary Core Ideas
- PS1.B Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.

