Item AP044006: Design an experiment to determine which water bottle design minimizes the amount of thermal energy that is transferred from the bottle’s contents to the surroundings.
Aluminum bottles are used every day to keep hot liquids from getting cold and to keep cold liquids from getting warm. Below is a photo of an aluminum bottle that contains hot coffee and a photo of an aluminum bottle that contains liquid nitrogen, a very cold liquid used by scientists.

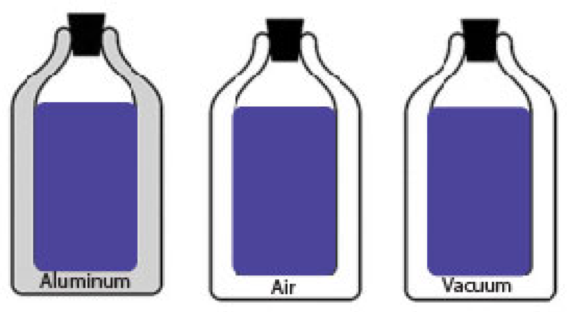
Aluminum bottles can be designed in different ways. Some aluminum bottles have walls made of solid aluminum, and some have walls that are hollow. Bottles that have hollow walls have the space between the walls filled with air or have had all the air removed. When all the air is removed so that there is no matter in the space, it is called a vacuum.
A biologist wants to move some bacteria in her lab to another lab. She doesn’t want the bacteria to get cold while she moves them. She decides to put them and the warm liquid in which they live into an aluminum bottle.
She is interested in testing the three different bottles to see which one does the best job of minimizing the transfer of thermal energy from the warm liquid inside the bottle to the cooler air outside the bottle.
1. First, the biologist thinks about how she could measure the transfer of thermal energy from a liquid inside the bottle to the air outside the bottle. She puts warm water in one bottle and leaves the bottle in a refrigerator for five minutes. Which of the following would the biologists have to measure to calculate the change in energy of the water inside the bottle? Choose all that apply.
A. The mass of the water
B. The temperature of the water before it was put in the refrigerator
C. The temperature of the water after it was left in refrigerator for five minutes
2. Next, she decides to design a controlled experiment that could help her figure out which of the three bottles would reduce the transfer of thermal energy the most. She has the following materials for her experiment:
- One of each of the three different aluminum bottles
- A refrigerator
- A scale
- A measuring cup
- Water
- A thermometer
Write a step-by-step procedure outlining a controlled experiment to determine which bottle reduces the transfer of thermal energy the most. Your procedure should include:
- Specific steps to follow
- What measurements should be made
- When measurements should be made
3. If you did your experiment and collected data, how could you use that data to figure out which bottle reduced the transfer of thermal energy the most?
4. How did you ensure that your investigation was a fair comparison of the different bottles?
5. Now the biologist starts to think about why the water inside the bottles cools down and the air around the bottles warms up. She knows that the main difference between the bottles is the number and type of molecules between the walls. So, she wants to focus her explanation on the movement of the molecules.
Write a molecular-level explanation for why the water inside the bottles cools down and the air around the bottles warms up. Explain these temperature changes using what you know about the relationships between temperature, the motion of molecules, and energy transfer.
6. After doing the experiment, the biologist finds that the bottle with the vacuum keeps liquids warmer than the solid aluminum bottle or the hollow bottle filled with air.
Why does the bottle with the vacuum keep the water inside the bottle warmer than the other bottles? Remember that the vacuum means that there are no molecules of air in the space between the walls of the bottle.
- Percent of Points Earned

- Points Earned
| Avg. Earned | Possible | Percent | |
|---|---|---|---|
| Q1 | 0.13 | 1 | 13% |
| Q2 | 1.36 | 3 | 45% |
| Q3 | 0.37 | 2 | 19% |
| Q4 | 0.5 | 1 | 50% |
| Q5 | 0.26 | 3 | 9% |
| Q6 | 0.33 | 3 | 11% |
- Overall Task Difficulty
| Total Points Earned | Total Points Possible | Total Percent | |
|---|---|---|---|
| 2.95 | 13 | 23% |
n = 281
Note: The total percent is a weighted average based on the total number of points earned divided by the total number of points possible.
- Science and Engineering Practices
- SEP3 Make predictions about what would happen if a variable changes.
SEP3 Plan an investigation individually and collaboratively, and in the design: identify independent and dependent variables and controls, what tools are needed to do the gathering, how measurements will be recorded, and how many data are needed to support a claim.
SEP6 Apply scientific ideas or principles to design, construct, and/or test a design of an object, tool, process or system. - Crosscutting Concepts
- CC5 The transfer of energy can be tracked as energy flows through a designed or natural system.
- Disciplinary Core Ideas
- PS3.A Temperature is a measure of the average kinetic energy of particles of matter. The relationship between the temperature and the total energy of a system depends on the types, states, and amounts of matter present.
PS3.B The amount of energy transfer needed to change the temperature of a matter sample by a given amount depends on the nature of the matter, the size of the sample, and the environment.
PS3.B Energy is spontaneously transferred out of hotter regions or objects and into colder ones.

