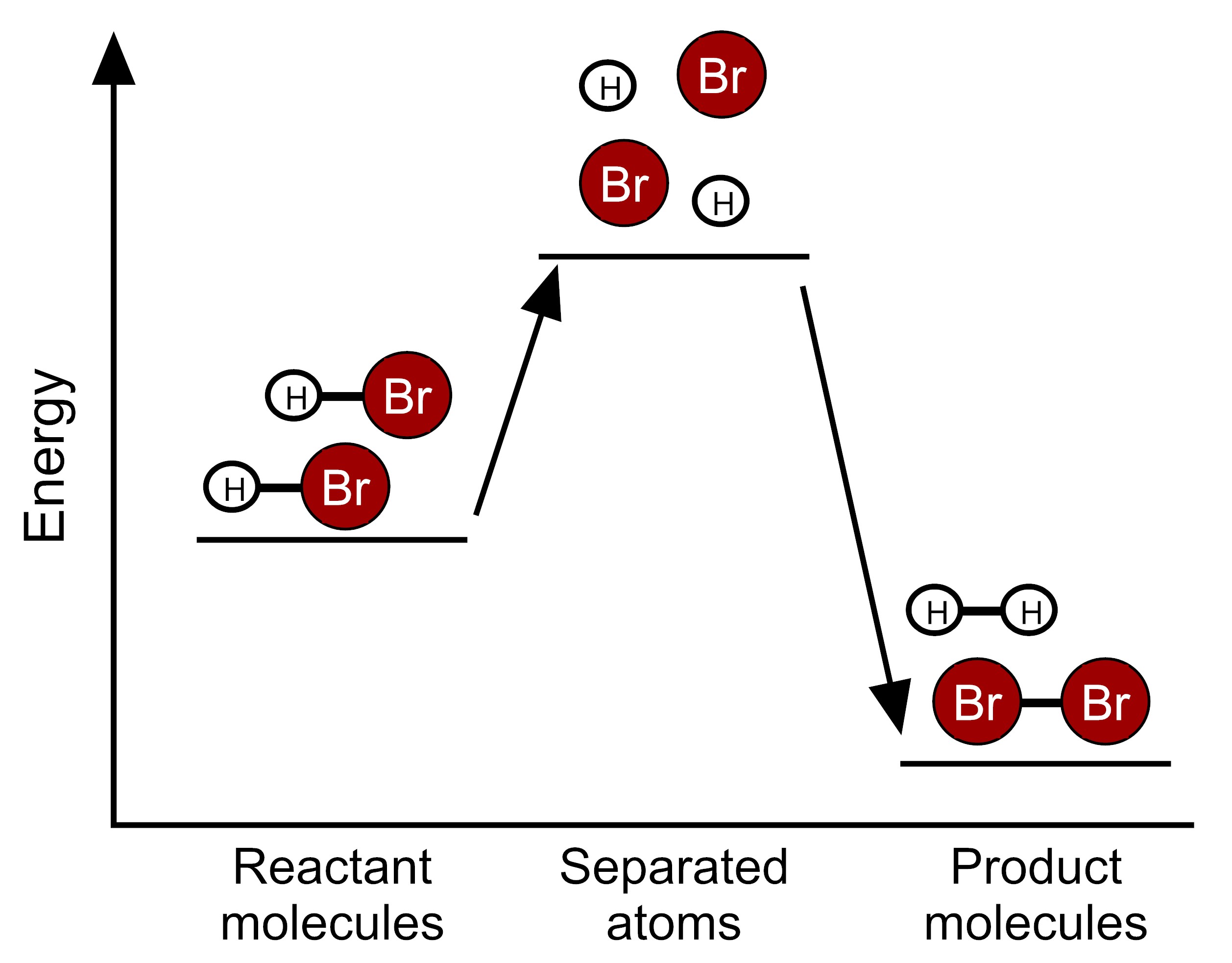
Item EC053002: The reaction between bromine and hydrogen takes in energy because the total bond energy of the reactants is greater than the total bond energy of the products. (This item uses molecular models and graphs.)
Two moles of hydrogen bromide molecules (HBr) react to form one mole of hydrogen molecules (H2) and one mole of bromine molecules (Br2) (A mole is a very large quantity of molecules). In the equation for this chemical reaction shown below, dark red circles labeled Br represent bromine atoms and white circles labeled H represent hydrogen atoms. Lines represent bonds between the atoms that make up molecules.
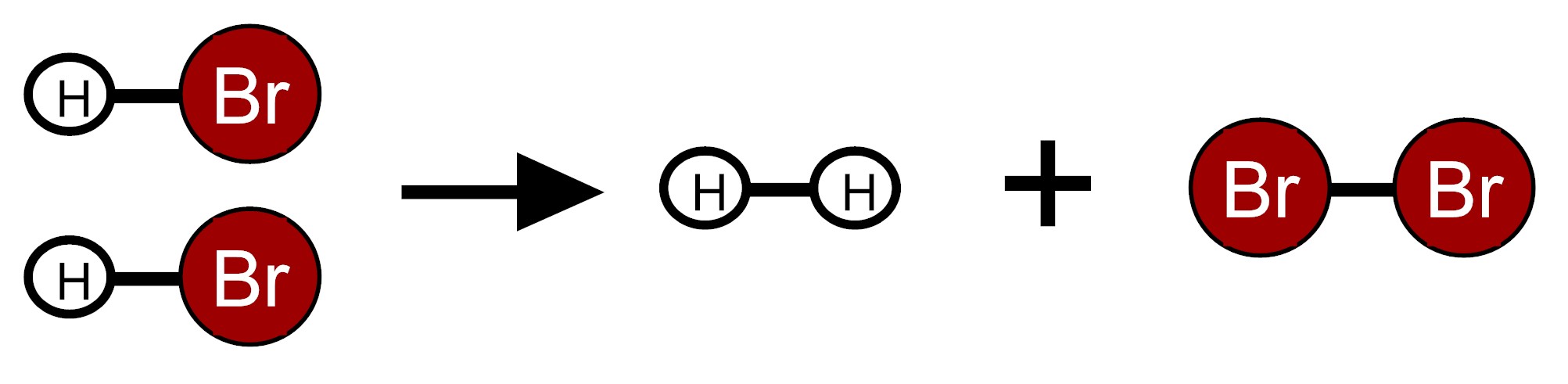
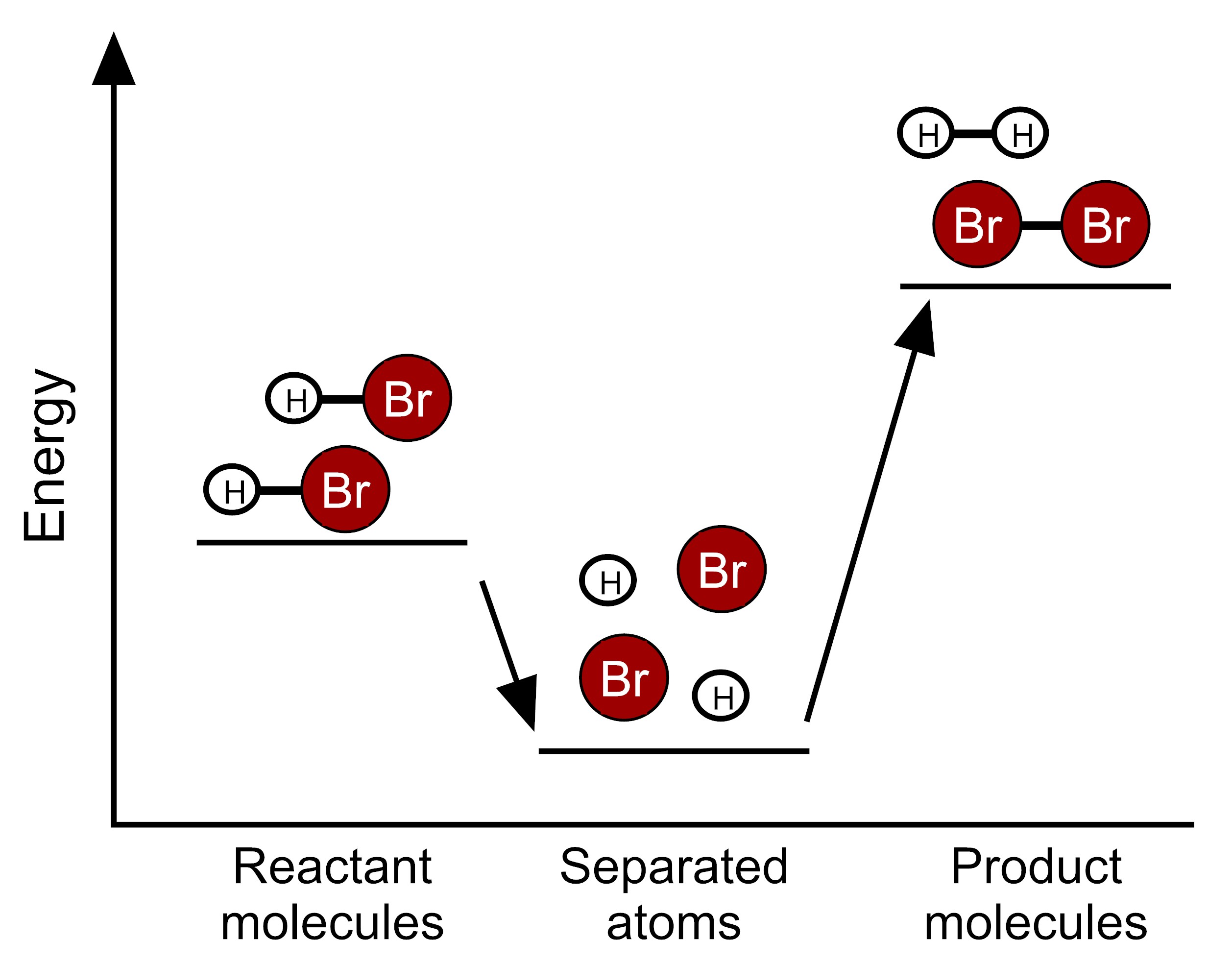
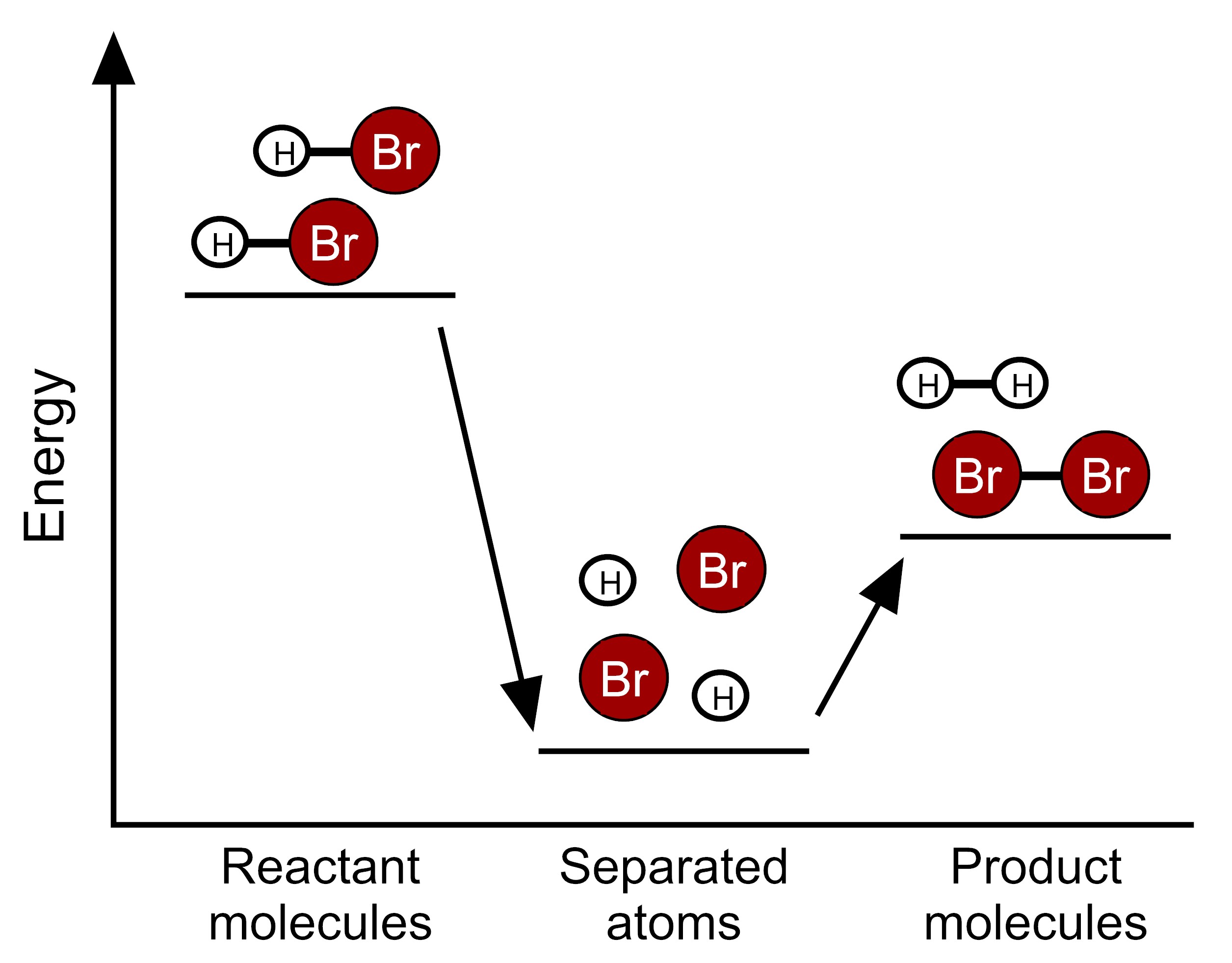
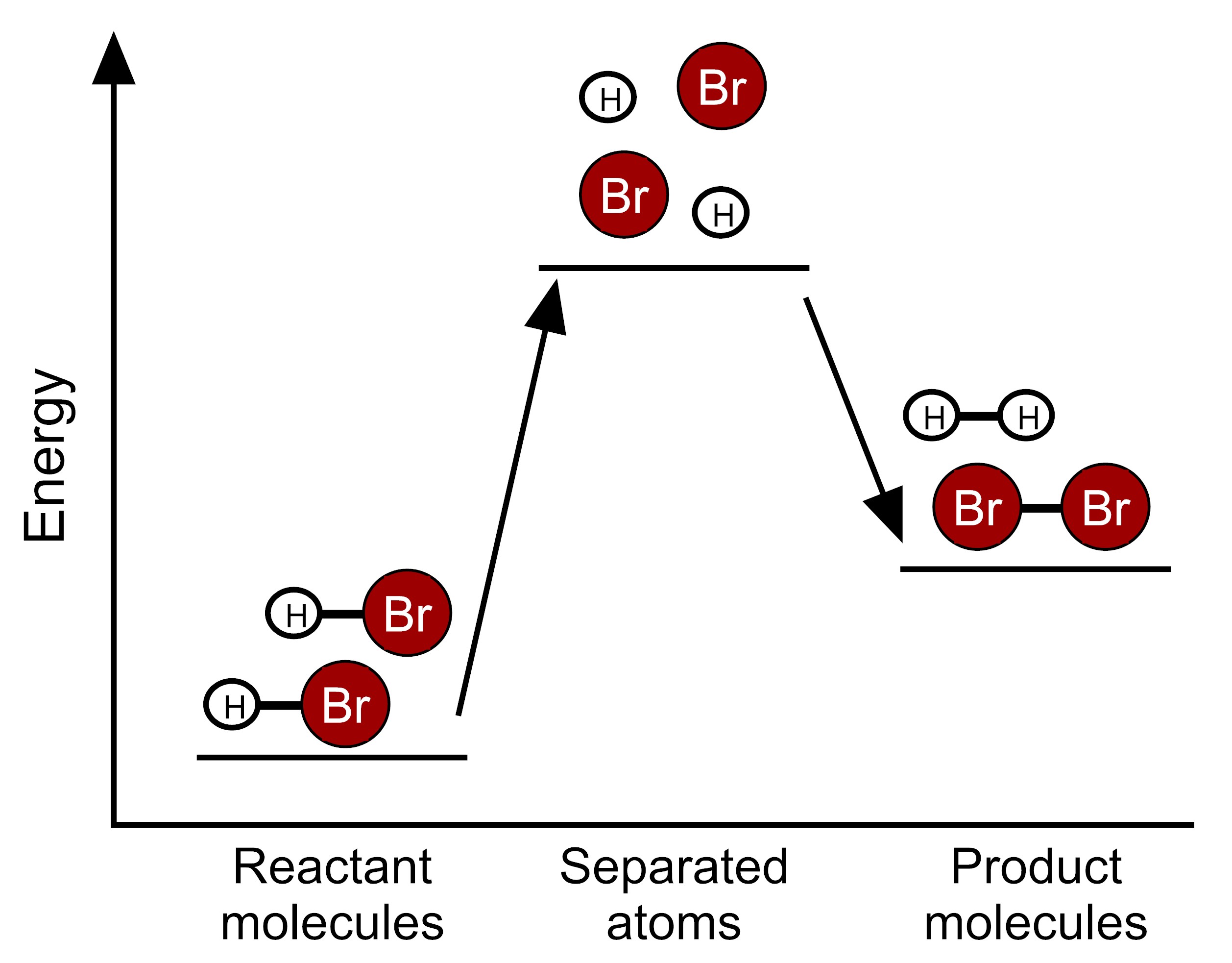
Scientists have determined the amount of energy associated with breaking and forming the bonds between the atoms that make up the molecules of HBr, H2, and Br2. We can use these bond energies to predict whether this reaction will release energy or take in energy. The table below show the total bond energy of two moles of HBr and the total bond energy of one mole of H2 plus one mole of Br2. [A kilojoule (kJ) is a unit of energy.]
| Total bond energies in kilojoules (kJ) | ||
| Reactants | 2 HBr | 732 kJ |
| Products | H2 + Br2 | 629 kJ |
Based on this information, which model represents the energy changes that occur during this reaction?
Pre-Test
Answer Choice |
Overall |
Grades |
Gender |
Primary Language |
||||
|---|---|---|---|---|---|---|---|---|
| n = 164 |
6–8 n = 0 |
9–12 n = 152 |
Male n = 84 |
Female n = 64 |
English n = 145 |
Other n = 7 |
||
| A. |
 |
20% | NAN% | 20% | 25% | 13% | 19% | 29% |
| B. |
 |
34% | NAN% | 34% | 27% | 42% | 34% | 43% |
| C. |
 |
21% | NAN% | 21% | 24% | 17% | 21% | 14% |
| D. |
 |
25% | NAN% | 25% | 24% | 28% | 26% | 14% |
Post-Test
Answer Choice |
Overall |
Grades |
Gender |
Primary Language |
||||
|---|---|---|---|---|---|---|---|---|
| n = 170 |
6–8 n = 0 |
9–12 n = 159 |
Male n = 100 |
Female n = 53 |
English n = 143 |
Other n = 12 |
||
| A. |
 |
12% | NAN% | 13% | 14% | 9% | 13% | 0% |
| B. |
 |
46% | NAN% | 45% | 46% | 47% | 43% | 83% |
| C. |
 |
17% | NAN% | 17% | 18% | 11% | 19% | 0% |
| D. |
 |
25% | NAN% | 25% | 22% | 32% | 25% | 17% |

