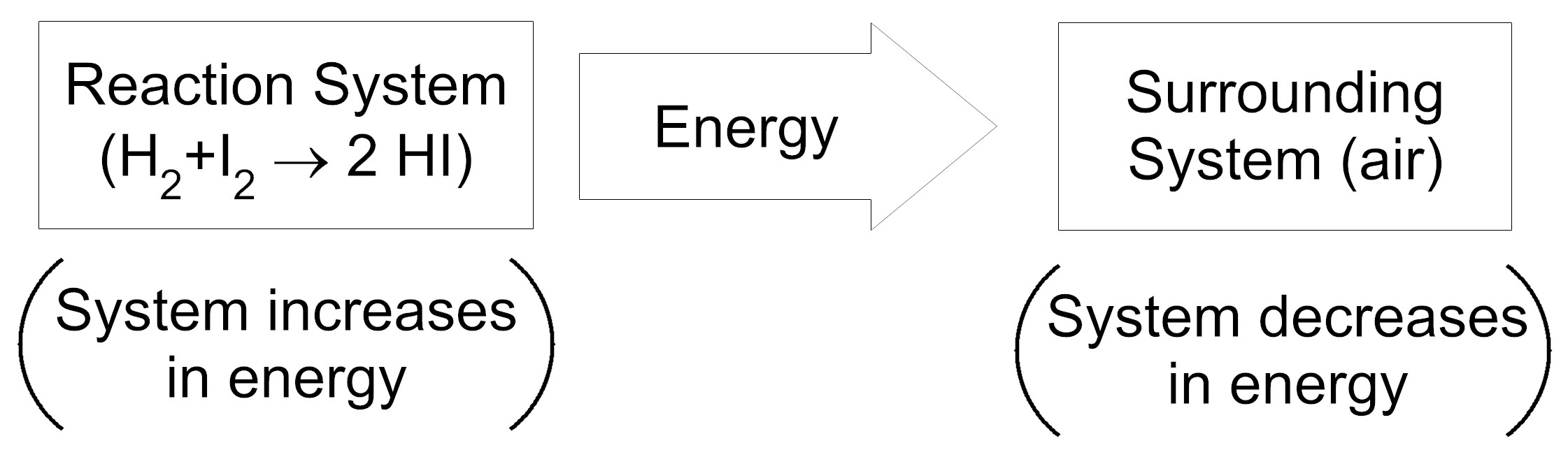
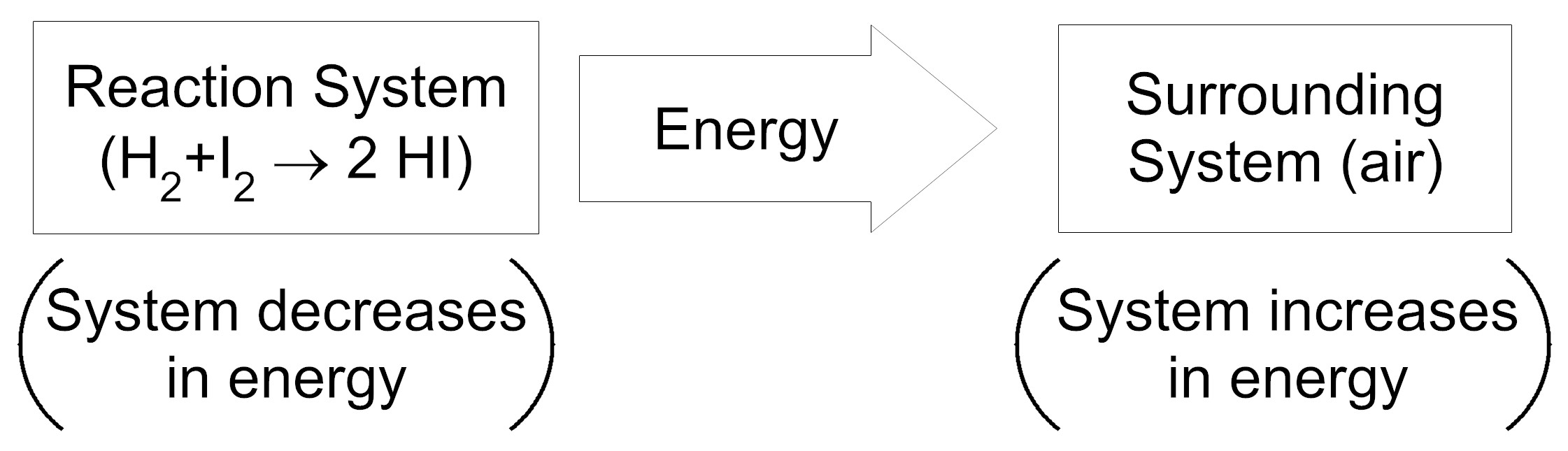
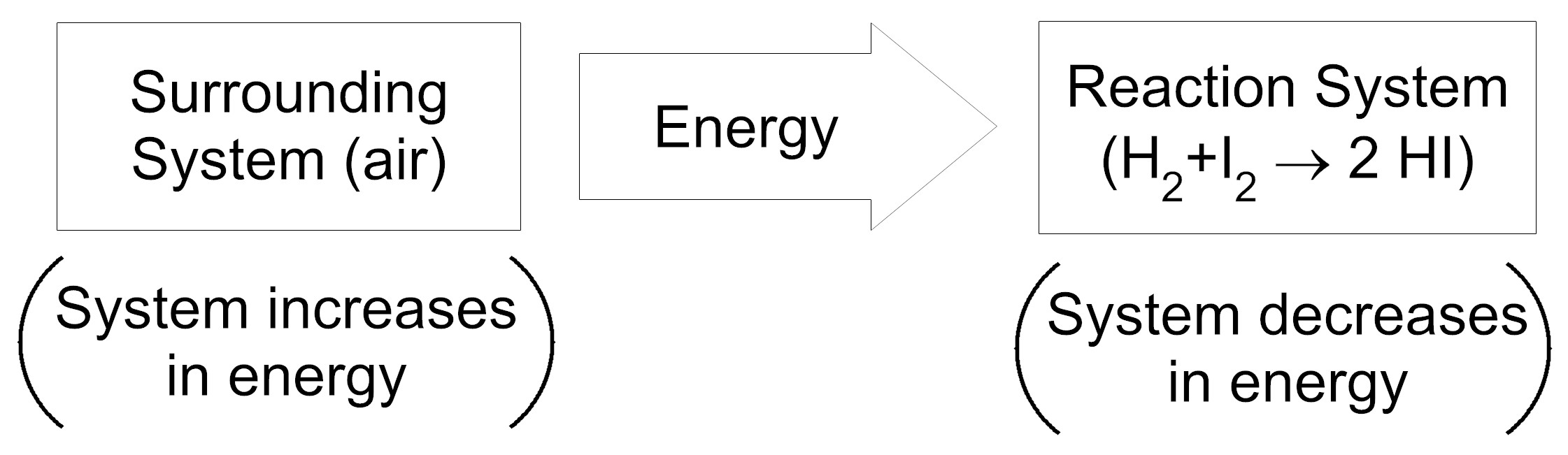
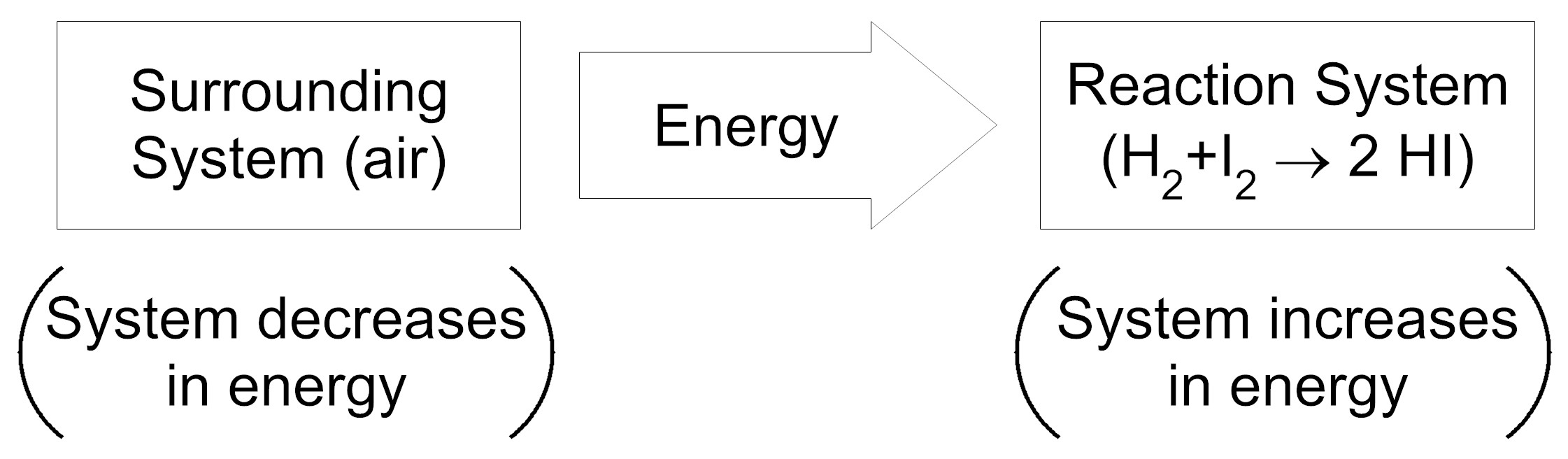
Item EB052002: During the reaction between hydrogen and iodine, energy is transferred from the reaction system to the surrounding system. (This item uses an energy transfer model.)
An energy transfer model shows the direction of energy transfer between two systems. Each box in the model represents a system and the thick arrow between the boxes represents the direction of energy transfer. In parentheses under each box, is a description of how the amount of energy in the system changes during the reaction.
Hydrogen (H2) and iodine (I2) can react to form hydrogen iodide (HI) as shown in the following reaction equation.
H2 + I2 → 2 HI
Scientists have determined the amount of energy associated with breaking and forming the bonds between the atoms that make up the molecules of H2, I2, and HI. The table below show the total bond energy of one mole of H2 plus one mole of I2 and the total bond energy of two moles of HI. [A kilocalorie (kcal) is a unit of energy and a mole is a very large quantity of molecules.]
| Total bond energies in kilocalories (kcal) | ||
| Reactants | H2 + I2 | 136 kcal |
| Products | 2 HI | 142 kcal |
Based on this information, which model represents the energy transfer between the reaction system (the system containing the chemical reaction) and the surrounding system (the air surrounding the reaction system)?
- Distribution of Responses

- Points Earned
| Group | Correct | Total | Percent |
|---|---|---|---|
| Overall | 47 | 127 | 37% |
| Grades | |||
| 6–8 | N/A | N/A | N/A |
| 9–12 | 42 | 113 | 37% |
| Gender | |||
| Male | 27 | 70 | 39% |
| Female | 12 | 40 | 30% |
| Primary Language | |||
| English | 40 | 109 | 37% |
| Other | 3 | 8 | 38% |
- Distribution of Responses

- Points Earned
| Group | Correct | Total | Percent |
|---|---|---|---|
| Overall | 64 | 144 | 44% |
| Grades | |||
| 6–8 | N/A | N/A | N/A |
| 9–12 | 54 | 126 | 43% |
| Gender | |||
| Male | 24 | 72 | 33% |
| Female | 29 | 53 | 55% |
| Primary Language | |||
| English | 50 | 120 | 42% |
| Other | 3 | 5 | 60% |
- Disciplinary Core Ideas
- PS1.A A stable molecule has less energy than the same set of atoms separated; one must provide at least this energy in order to take the molecule apart.
PS1.B Chemical processes, their rates, and whether or not energy is stored or released can be understood in terms of the collisions of molecules and the rearrangements of atoms into new molecules, with consequent changes in the sum of all bond energies in the set of molecules that are matched by changes in kinetic energy.