Item EC007003: The reaction between methanol and oxygen releases energy because the amount of energy released when bonds form is greater than the amount of energy required to break bonds.
Methanol (CH3OH) reacts with oxygen (O2) to form carbon dioxide (CO2) and water (H2O). Molecular models of the molecules involved in this reaction are shown below. In the models, gray circles represent carbon atoms, red circles represent oxygen atoms, and white circles represent hydrogen atoms. Lines represent bonds between the atoms that make up molecules.
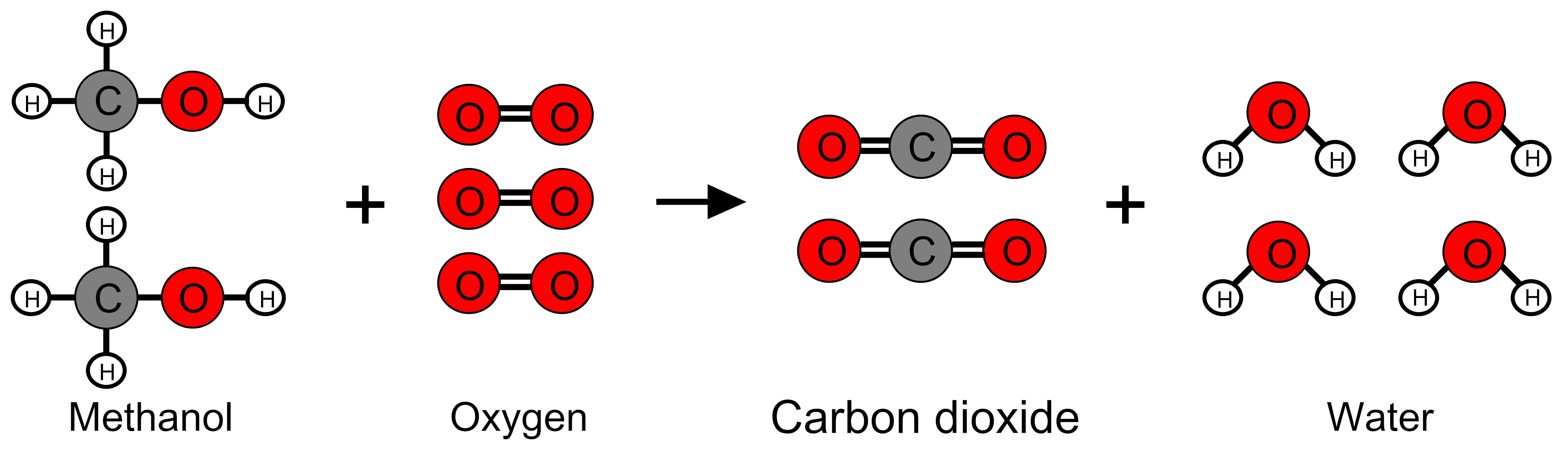
During this reaction, a flame is observed and the temperature of the surroundings increases. Scientists have determined the amount of energy associated with the breaking and forming bonds of the reactant and product molecules. The table below lists the sum of the bond energies for all the bonds in the reactant molecules and for all the bonds in the product molecules when two moles of methanol react with three moles of oxygen. [A kilocalorie (kcal) is a unit of energy and a mole is a very large quantity of molecules.]
| Sum of the bond energies in kilocalories (kcal) | ||
| Reactants | 2 CH3OH + 3 O2 | 1,345 kcal |
| Products | 2 CO2 + 4 H2O | 1,651 kcal |
Does this chemical reaction give off energy or take in energy? Support your answer using the models and data given above. In your response, be sure to describe the changes in both matter and energy.
Pre-Test
Overall |
Grades |
Gender |
Primary Language |
||||
|---|---|---|---|---|---|---|---|
| n = 2675 |
6–8 n = 0 |
9–12 n = 2360 |
Male n = 1390 |
Female n = 990 |
English n = 2235 |
Other n = 150 |
|
| 4% | N/A | 4% | 5% | 3% | 4% | 5% | |
Post-Test
Overall |
Grades |
Gender |
Primary Language |
||||
|---|---|---|---|---|---|---|---|
| n = 2675 |
6–8 n = 0 |
9–12 n = 2445 |
Male n = 1405 |
Female n = 1000 |
English n = 2260 |
Other n = 155 |
|
| 8% | N/A | 9% | 8% | 9% | 9% | 6% | |

