Item SB046002: The characteristic properties of a substance are determined by the number and types of atoms and the way the atoms are arranged to form the molecules of the substance, therefore, two substances with different arrangements of atoms will not have the same characteristic properties.
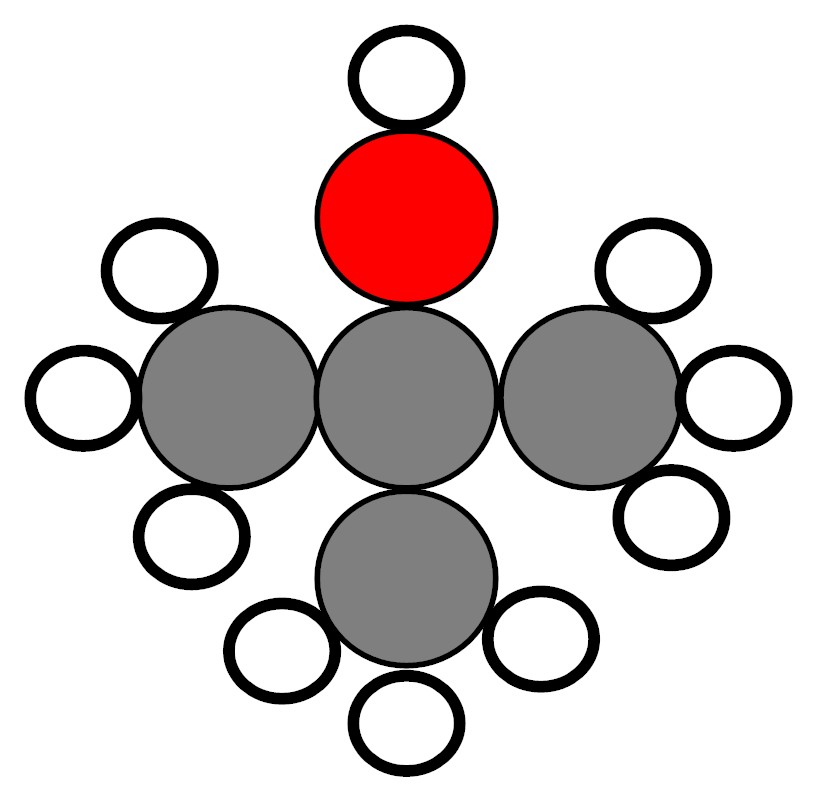
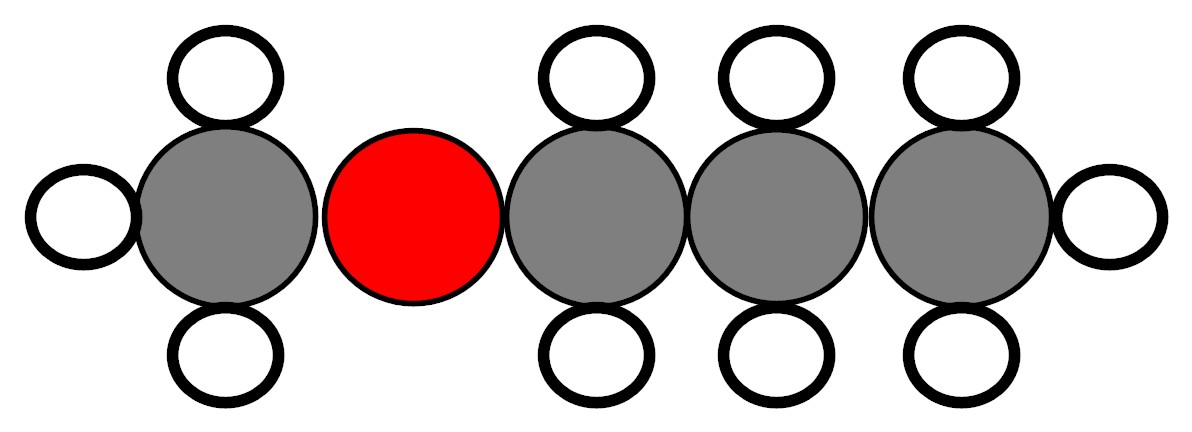
A student has two containers. Each container has a colorless liquid inside of it. The liquids are pure substances. His teacher shows him models of the molecules that make up each of the liquids. Both of the molecules are made up of 4 carbon atoms, 10 hydrogen atoms, and 1 oxygen atom. However, the way the atoms are arranged to form the molecules of Liquid 1 is different from the way the atoms are arranged to form the molecules of Liquid 2.
|
|
|
Model of the molecules
that make up Liquid 1
|
Model of the molecules
that make up Liquid 2
|
The teacher asks the student to predict whether or not the two colorless liquids will have the same set of characteristic properties (for example, density, melting point, and boiling point). Will the liquids have the same set of characteristic properties? Why or why not?
- Yes, because the characteristic properties of a substance are determined only by the types of atoms, and the two molecules are both made up of carbon, hydrogen, and oxygen atoms.
- Yes, because the characteristic properties of a substance are determined by the number and types of atoms, and the two molecules are both made up of 4 carbon atoms, 10 hydrogen atoms, and 1 oxygen atom.
- No, because the characteristic properties of a substance are determined by the number and types of atoms and the way the atoms are arranged to form the molecules of the substance, and the atoms are arranged in different ways in the two molecules.
- More information is needed because the characteristic properties of a substance, such as density, melting point, and boiling point are determined by something other than the number and types of atoms and the way the atoms are arranged to form the molecules of the substance.
Pre-Test
Answer Choice |
Overall |
Grades |
Gender |
Primary Language |
||||
|---|---|---|---|---|---|---|---|---|
| n = 372 |
6–8 n = 372 |
9–12 n = 0 |
Male n = 204 |
Female n = 168 |
English n = 345 |
Other n = 27 |
||
| A. | Yes, because the characteristic properties of a substance are determined only by the types of atoms, and the two molecules are both made up of carbon, hydrogen, and oxygen atoms. | 12% | 12% | NAN% | 12% | 13% | 13% | 4% |
| B. | Yes, because the characteristic properties of a substance are determined by the number and types of atoms, and the two molecules are both made up of 4 carbon atoms, 10 hydrogen atoms, and 1 oxygen atom. | 30% | 30% | NAN% | 29% | 31% | 29% | 41% |
| C. | No, because the characteristic properties of a substance are determined by the number and types of atoms and the way the atoms are arranged to form the molecules of the substance, and the atoms are arranged in different ways in the two molecules. | 41% | 41% | NAN% | 41% | 40% | 41% | 41% |
| D. | More information is needed because the characteristic properties of a substance, such as density, melting point, and boiling point are determined by something other than the number and types of atoms and the way the atoms are arranged to form the molecules of the substance. | 17% | 17% | NAN% | 17% | 17% | 17% | 15% |
Post-Test
Answer Choice |
Overall |
Grades |
Gender |
Primary Language |
||||
|---|---|---|---|---|---|---|---|---|
| n = 373 |
6–8 n = 373 |
9–12 n = 0 |
Male n = 204 |
Female n = 169 |
English n = 345 |
Other n = 28 |
||
| A. | Yes, because the characteristic properties of a substance are determined only by the types of atoms, and the two molecules are both made up of carbon, hydrogen, and oxygen atoms. | 5% | 5% | NAN% | 4% | 6% | 5% | 11% |
| B. | Yes, because the characteristic properties of a substance are determined by the number and types of atoms, and the two molecules are both made up of 4 carbon atoms, 10 hydrogen atoms, and 1 oxygen atom. | 24% | 24% | NAN% | 23% | 25% | 24% | 29% |
| C. | No, because the characteristic properties of a substance are determined by the number and types of atoms and the way the atoms are arranged to form the molecules of the substance, and the atoms are arranged in different ways in the two molecules. | 61% | 61% | NAN% | 63% | 59% | 62% | 50% |
| D. | More information is needed because the characteristic properties of a substance, such as density, melting point, and boiling point are determined by something other than the number and types of atoms and the way the atoms are arranged to form the molecules of the substance. | 10% | 10% | NAN% | 10% | 10% | 10% | 11% |

