Item SB057001: When nitric acid and copper react, the atoms detach from one another and then link together in different ways to make the molecules of the red gas and green liquid.
As part of an experiment in science class, a student placed a piece of copper, which is a reddish-orange solid, and some nitric acid, which is a colorless liquid, into a container and sealed it. A chemical reaction occurred, and the student saw a green liquid and a red gas form in the container.
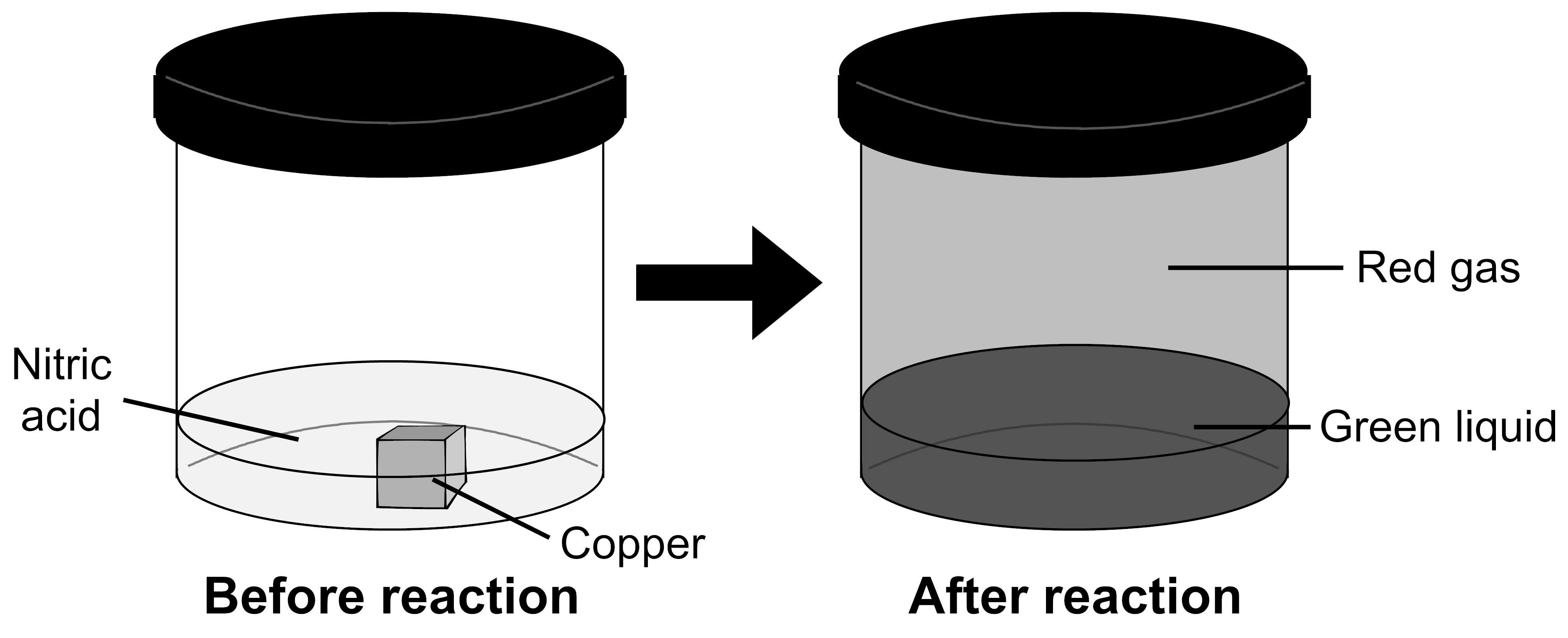
What happened to the atoms that made up the copper and nitric acid?
- The atoms broke down to release the molecules of the red gas and green liquid.
- The atoms detached from one another and then linked together in different ways to make the molecules of the red gas and green liquid.
- The atoms turned into different atoms and then connected to form the molecules of the red gas and green liquid.
- Nothing happened to the atoms. The red gas and green liquid are made up of the same molecules as the nitric acid and copper, but the molecules are different colors.
- Distribution of Responses

- Points Earned
| Group | Correct | Total | Percent |
|---|---|---|---|
| Overall | 143 | 359 | 40% |
| Grades | |||
| 6–8 | 143 | 359 | 40% |
| 9–12 | N/A | N/A | N/A |
| Gender | |||
| Male | 77 | 197 | 39% |
| Female | 66 | 162 | 41% |
| Primary Language | |||
| English | 138 | 331 | 42% |
| Other | 5 | 28 | 18% |
- Distribution of Responses

- Points Earned
| Group | Correct | Total | Percent |
|---|---|---|---|
| Overall | 261 | 349 | 75% |
| Grades | |||
| 6–8 | 261 | 349 | 75% |
| 9–12 | N/A | N/A | N/A |
| Gender | |||
| Male | 143 | 187 | 76% |
| Female | 118 | 162 | 73% |
| Primary Language | |||
| English | 249 | 322 | 77% |
| Other | 12 | 27 | 44% |

