Item RG155002: As water at the bottom of a jar gets warmer than the water above it, the water at the bottom of the jar and its thermal energy both rise because thermal energy is associated with the movement of the water molecules.
A jar of cold water is placed over a flame, and the flame warms the water at the bottom of the jar, increasing its thermal energy.
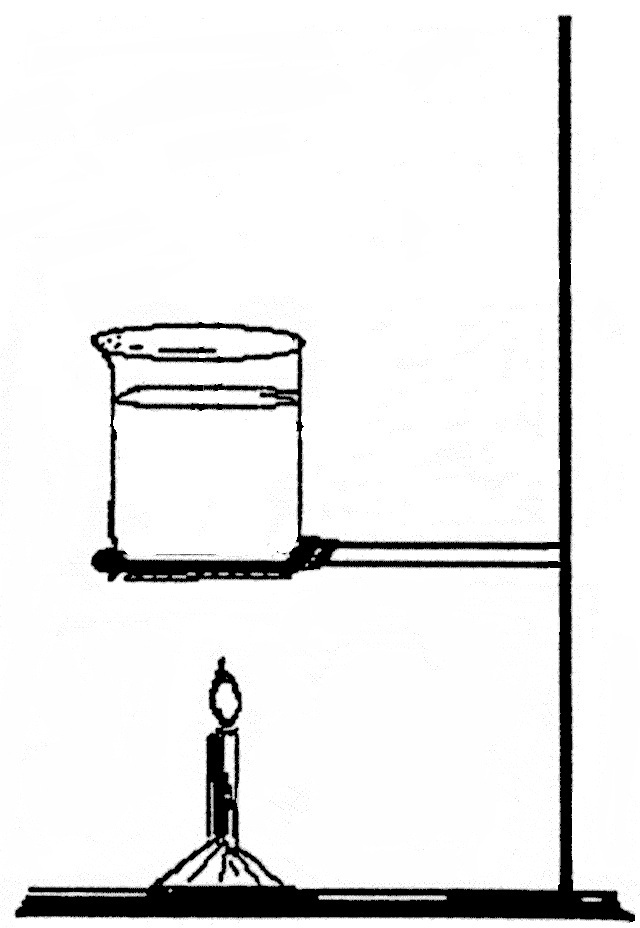
What happens to the water at the bottom of the jar and to the thermal energy of the water?
- As the water at the bottom of the jar gets warmer than the water above it, the water at the bottom of the jar and its thermal energy both rise because thermal energy is associated with the movement of the water molecules.
- Nothing moves until the water starts to boil, and then the water at the bottom of the jar and its thermal energy both rise because thermal energy is associated with the movement of the water molecules.
- As the water at the bottom of the jar gets warmer than the water above it, the thermal energy of the water at the bottom of the jar rises, but the water itself does not rise because thermal energy is not associated with the movement of the water molecules.
- As the water at the bottom of the jar gets warmer than the water above it, the water at the bottom of the jar and its thermal energy both rise, but they rise separately because thermal energy is not associated with the movement of the water molecules.
Answer Choice |
Overall |
Grades |
Gender |
Primary Language |
||||
|---|---|---|---|---|---|---|---|---|
| n = 1451 |
4–5 n = 0 | 6–8 n = 799 |
9–12 n = 652 |
Male n = 723 |
Female n = 707 |
English n = 1290 |
Other n = 136 |
|
| A. | 52% | N/A | 50% | 54% | 53% | 51% | 52% | 51% |
| B. | 16% | N/A | 17% | 15% | 17% | 15% | 16% | 17% |
| C. | 22% | N/A | 23% | 21% | 19% | 25% | 23% | 15% |
| D. | 10% | N/A | 11% | 9% | 11% | 9% | 9% | 17% |

