Item EG084002: For two glasses containing water at the same temperature, the glass with more water has more thermal energy than the glass with less water.
A student has two identical glasses that have different amounts of water in them. Glass 1 has more water in it than Glass 2.
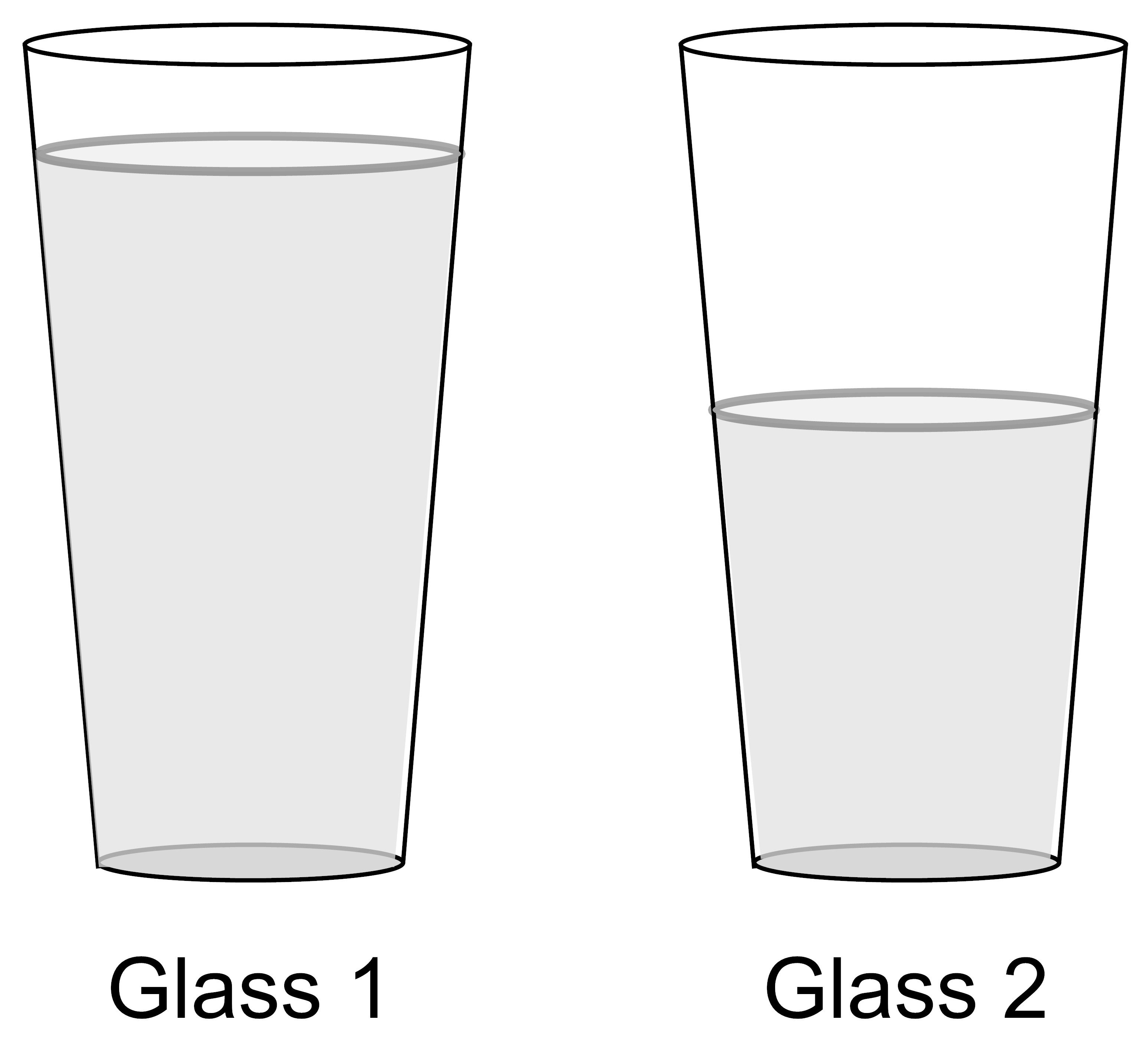
If the temperature of the water in the glasses is the same, how does the thermal energy of Glass 1 compare to the thermal energy of Glass 2?
- Glass 1 has more thermal energy than Glass 2.
- Glass 1 has less thermal energy than Glass 2.
- Glass 1 has the same amount of thermal energy as Glass 2.
- Neither glass of water has any thermal energy.
Answer Choice |
Overall |
Grades |
Gender |
Primary Language |
|||||
|---|---|---|---|---|---|---|---|---|---|
| n = 1462 |
4–5 n = 312 | 6–8 n = 598 |
9–12 n = 552 |
Male n = 720 |
Female n = 710 |
English n = 1281 |
Other n = 148 |
||
| A. | Glass 1 has more thermal energy than Glass 2. | 41% | 49% | 39% | 38% | 39% | 43% | 41% | 39% |
| B. | Glass 1 has less thermal energy than Glass 2. | 20% | 23% | 21% | 16% | 18% | 22% | 19% | 24% |
| C. | Glass 1 has the same amount of thermal energy as Glass 2. | 31% | 14% | 33% | 39% | 35% | 27% | 31% | 30% |
| D. | Neither glass of water has any thermal energy. | 8% | 13% | 7% | 7% | 8% | 8% | 8% | 7% |

