Item RG210001: If the chemical energy of the products of a chemical reaction is greater than the chemcial energy of the reactants, energy was taken in from the surroundings.
The graph below represents the amount of chemical energy in a system of reactants before a chemical reaction occurs and the amount of chemical energy in the system of products after the reaction occurs.
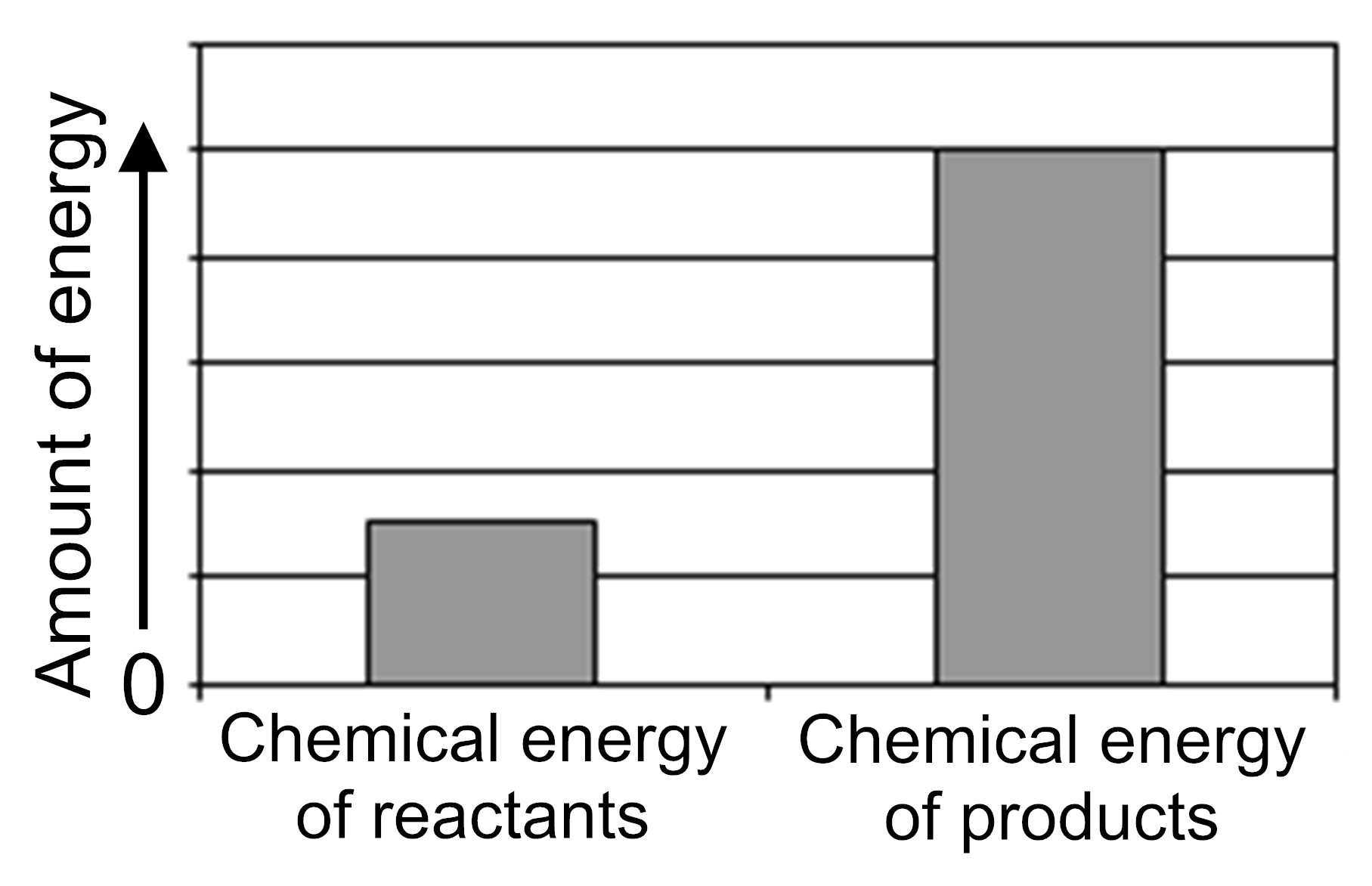
Is energy released to the surroundings or taken in from the surroundings during this reaction?
- Energy is released to the surroundings.
- Energy is taken in from the surroundings.
- Energy is neither taken in nor released.
- Whether energy is taken in or released cannot be determined from the graph.
- Distribution of Responses

- Scale Score for Item Difficulty
(200[Easy]-800[Difficult]) - 504
- Students Responding Correctly
| Group | Correct | Total | Percent |
|---|---|---|---|
| Overall | 626 | 1501 | 42% |
| Grades | |||
| 4–5 | 44 | 112 | 39% |
| 6–8 | 325 | 813 | 40% |
| 9–12 | 257 | 576 | 45% |
| Gender | |||
| Male | 286 | 689 | 42% |
| Female | 322 | 779 | 41% |
| Primary Language | |||
| English | 576 | 1367 | 42% |
| Other | 32 | 101 | 32% |
- Disciplinary Core Ideas
- PS1.B Some chemical reactions release energy, others store energy.

