Item RG207001: When comparing two ice cubes that are at the same temperature but are made up of different numbers of water molecules, the ice cube that is made up of more molecules has more thermal energy.
A student has two ice cubes. Ice Cube 1 and Ice Cube 2 are at the same temperature but Ice Cube 1 is made up of more water molecules than Ice Cube 2.
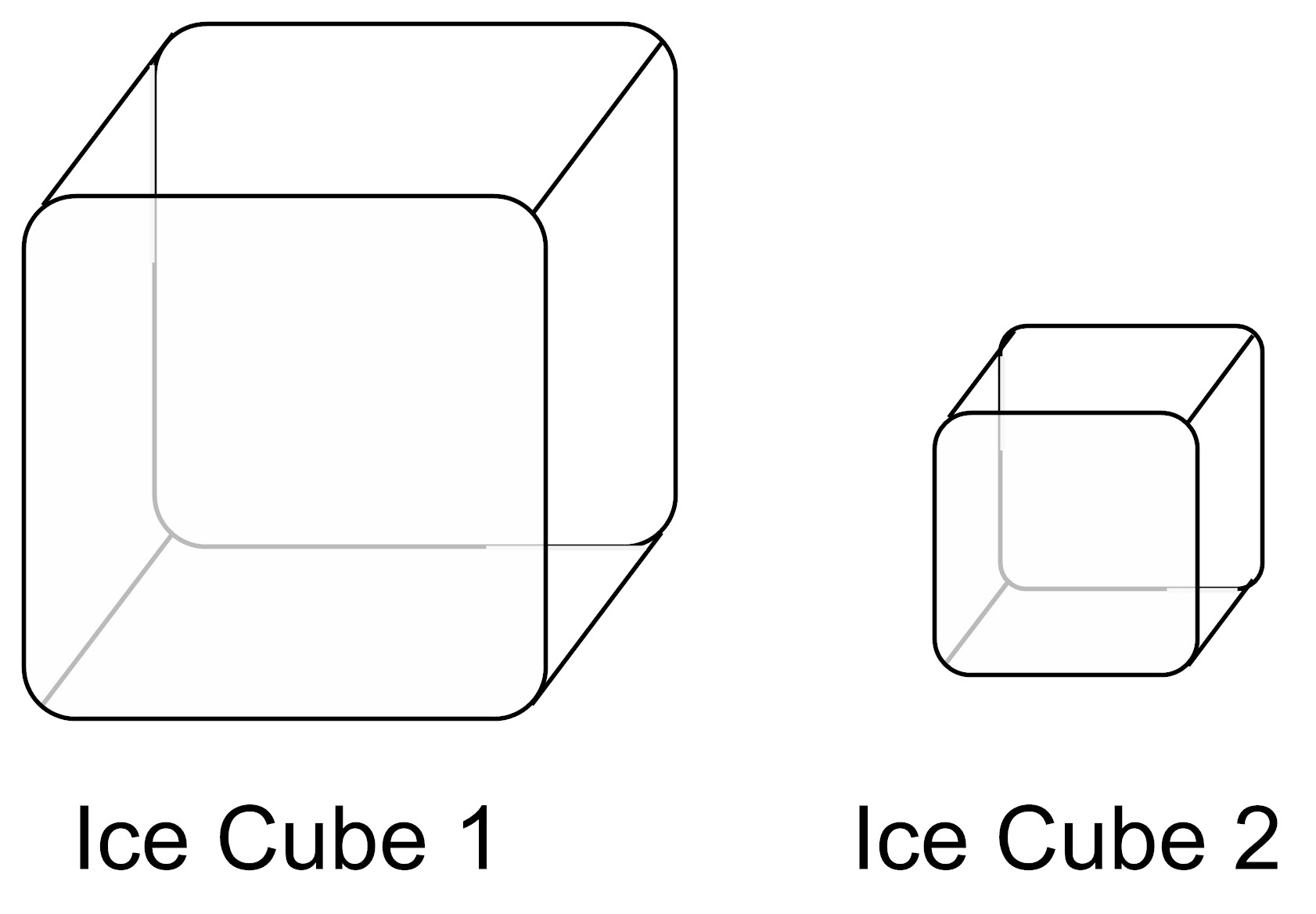
Which ice cube has more thermal energy and why?
- Ice Cube 1 has more thermal energy because it is made up of more molecules than Ice Cube 2.
- Ice Cube 2 has more thermal energy because it is made up of fewer molecules than Ice Cube 2.
- Ice Cube 1 and Ice Cube 2 have the same amount of thermal energy because they are at the same temperature.
- Ice Cube 1 and Ice Cube 2 do not have any thermal energy because frozen things do not have thermal energy.
- Distribution of Responses

- Scale Score for Item Difficulty
(200[Easy]-800[Difficult]) - 508
- Students Responding Correctly
| Group | Correct | Total | Percent |
|---|---|---|---|
| Overall | 441 | 1077 | 41% |
| Grades | |||
| 4–5 | N/A | N/A | N/A |
| 6–8 | 281 | 652 | 43% |
| 9–12 | 160 | 425 | 38% |
| Gender | |||
| Male | 221 | 545 | 41% |
| Female | 209 | 515 | 41% |
| Primary Language | |||
| English | 387 | 923 | 42% |
| Other | 43 | 137 | 31% |
- Disciplinary Core Ideas
- PS3.A Temperature is a measure of the average kinetic energy of particles of matter. The relationship between the temperature and the total energy of a system depends on the types, states, and amounts of matter present.
- NRC Framework
- PS3.A Thermal energy is the random motion of particles (whether vibrations in solid matter or molecules or free motion in a gas)...

