Item RG056003: A hotter tea kettle has more energy than a cooler tea kettel because the hotter tea kettle has a higher temperature.
An adult wants to heat up some water to make hot tea. He fills two tea kettles that are exactly the same with water and places them on the stove. While the kettles are on the stove, the temperature of the kettles and the water increases. When the kettles are very hot, the adult measures the temperature of each kettle. He finds that one kettle is hotter than the other.
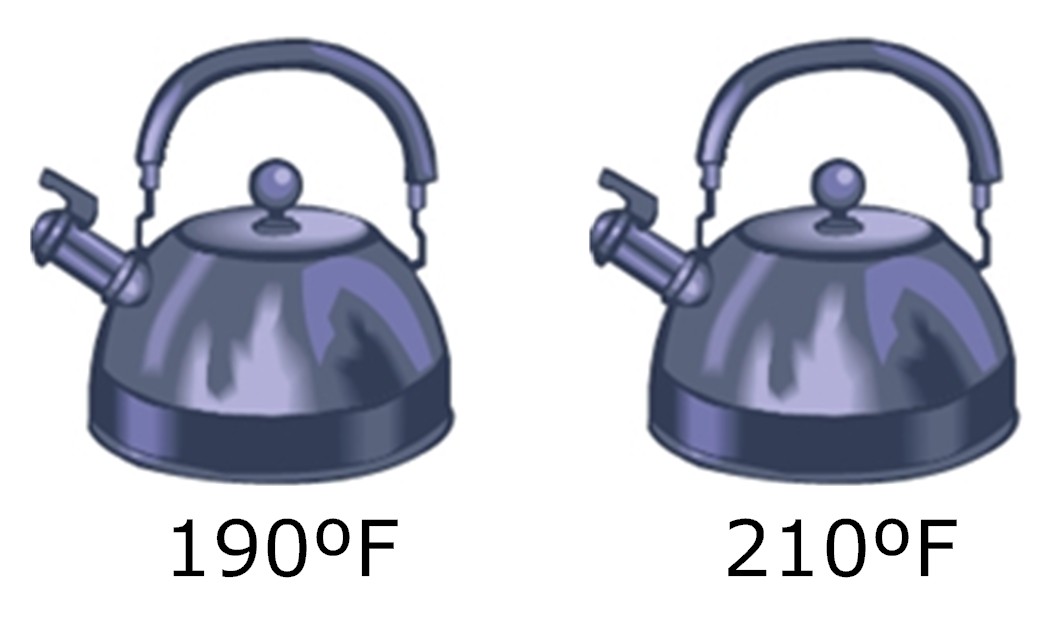
Which tea kettle has more energy?
- The hotter kettle has more energy because it has a higher temperature.
- The cooler kettle has more energy because it hasn’t used up as much energy as the hotter kettle.
- The kettles have the same amount of energy because they are both very hot.
- The kettles have the same amount of energy because how much energy something has is not related to how hot it is.
- Distribution of Responses

- Scale Score for Item Difficulty
(200[Easy]-800[Difficult]) - 449
- Students Responding Correctly
| Group | Correct | Total | Percent |
|---|---|---|---|
| Overall | 3353 | 4953 | 68% |
| Grades | |||
| 4–5 | 1129 | 1653 | 68% |
| 6–8 | 1139 | 1683 | 68% |
| 9–12 | 1085 | 1617 | 67% |
| Gender | |||
| Male | 1550 | 2283 | 68% |
| Female | 1722 | 2543 | 68% |
| Primary Language | |||
| English | 3064 | 4476 | 68% |
| Other | 202 | 336 | 60% |
- Disciplinary Core Ideas
- PS3.A Temperature is a measure of the average kinetic energy of particles of matter. The relationship between the temperature and the total energy of a system depends on the types, states, and amounts of matter present.
- NRC Framework
- PS3.A Thermal energy is the random motion of particles (whether vibrations in solid matter or molecules or free motion in a gas)...

