Item EG053006: For two ice cubes that are made up of the same number of molecules, the ice cube with the molecules moving at the lower average speed has less thermal energy.
A student has two ice cubes. Ice Cube 1 and Ice Cube 2 are both made up of the same number of molecules. The average speed of the molecules of Ice Cube 1 is less than the average speed of the molecules of Ice Cube 2.
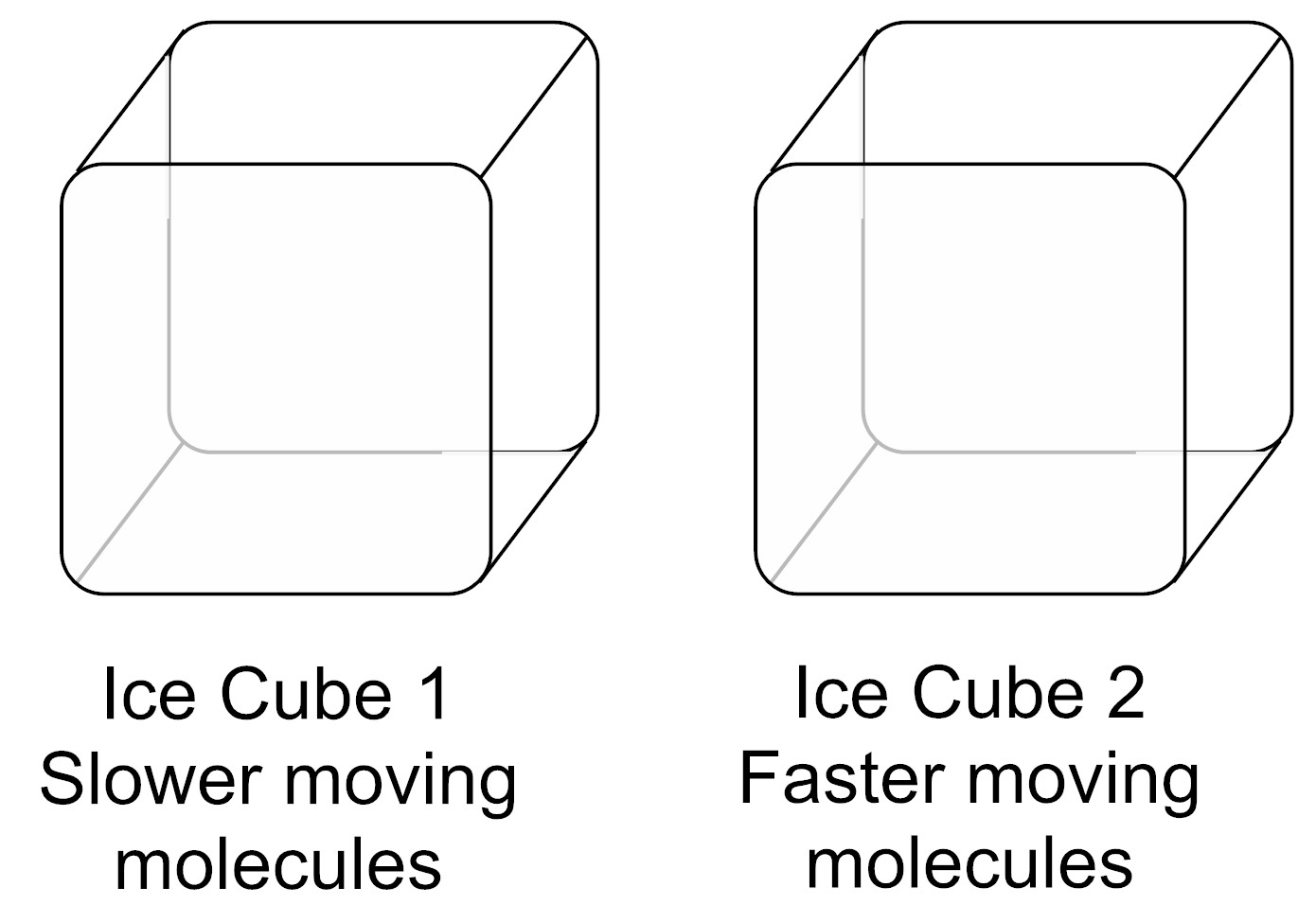
Which ice cube has less thermal energy and why?
- Ice Cube 1 has less thermal energy because its molecules are moving slower than the molecules of Ice Cube 2.
- Ice Cube 2 has less thermal energy because its molecules are moving faster than the molecules of Ice Cube 1.
- Ice Cube 1 and Ice Cube 2 have the same amount of thermal energy because anything made of ice has the same amount of thermal energy no matter how fast the molecules are moving.
- Ice Cube 1 and Ice Cube 2 do not have any thermal energy because frozen things do not have thermal energy.
- Distribution of Responses

- Scale Score for Item Difficulty
(200[Easy]-800[Difficult]) - 474
- Students Responding Correctly
| Group | Correct | Total | Percent |
|---|---|---|---|
| Overall | 634 | 1073 | 59% |
| Grades | |||
| 4–5 | N/A | N/A | N/A |
| 6–8 | 359 | 647 | 55% |
| 9–12 | 275 | 426 | 65% |
| Gender | |||
| Male | 314 | 543 | 58% |
| Female | 308 | 514 | 60% |
| Primary Language | |||
| English | 555 | 920 | 60% |
| Other | 67 | 137 | 49% |
- Disciplinary Core Ideas
- PS3.A Temperature is a measure of the average kinetic energy of particles of matter. The relationship between the temperature and the total energy of a system depends on the types, states, and amounts of matter present.
- NRC Framework
- Thermal energy is the random motion of particles (whether vibrations in solid matter or molecules or free motion in a gas)...

