Item SC090003: The number of each kind of atom stays the same during the reaction between copper and oxygen. (This item uses circles to represent atoms.)
When heated, oxygen reacts with copper to form copper oxide.
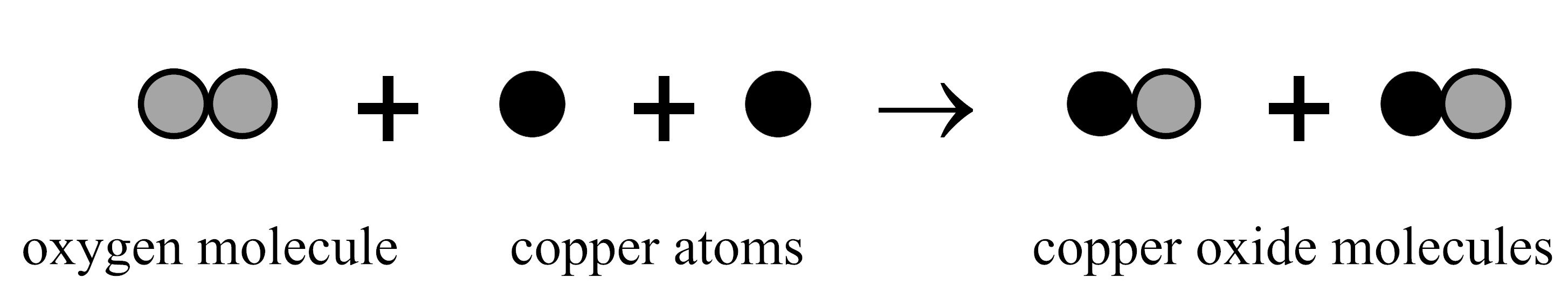
If this reaction occurs in a sealed container, will the mass of the container and everything in it increase, decrease, or stay the same and why?
- The mass will stay the same because the number of each kind of atom stays the same.
- The mass will decrease because two substances combine to form one substance.
- The mass will increase because a new kind of molecule is formed.
- More information is needed to tell if the mass will change.
Answer Choice |
Overall |
Grades |
Gender |
Primary Language |
||||
|---|---|---|---|---|---|---|---|---|
| n = 3879 |
6–8 n = 1611 |
9–12 n = 2251 |
Male n = 1914 |
Female n = 1887 |
English n = 3395 |
Other n = 397 |
||
| A. | The mass will stay the same because the number of each kind of atom stays the same. | 47% | 37% | 54% | 48% | 46% | 48% | 36% |
| B. | The mass will decrease because two substances combine to form one substance. | 15% | 16% | 14% | 14% | 15% | 14% | 16% |
| C. | The mass will increase because a new kind of molecule is formed. | 24% | 30% | 19% | 23% | 25% | 23% | 29% |
| D. | More information is needed to tell if the mass will change. | 15% | 17% | 13% | 15% | 15% | 15% | 18% |

