Item NG101001: When a warm can of soda is in contact with cold water, thermal energy is transferred from the can of soda to the water so the can of soda gets cooler and the water gets warmer.
A student places a warm can of soda into a bucket filled with cold water.
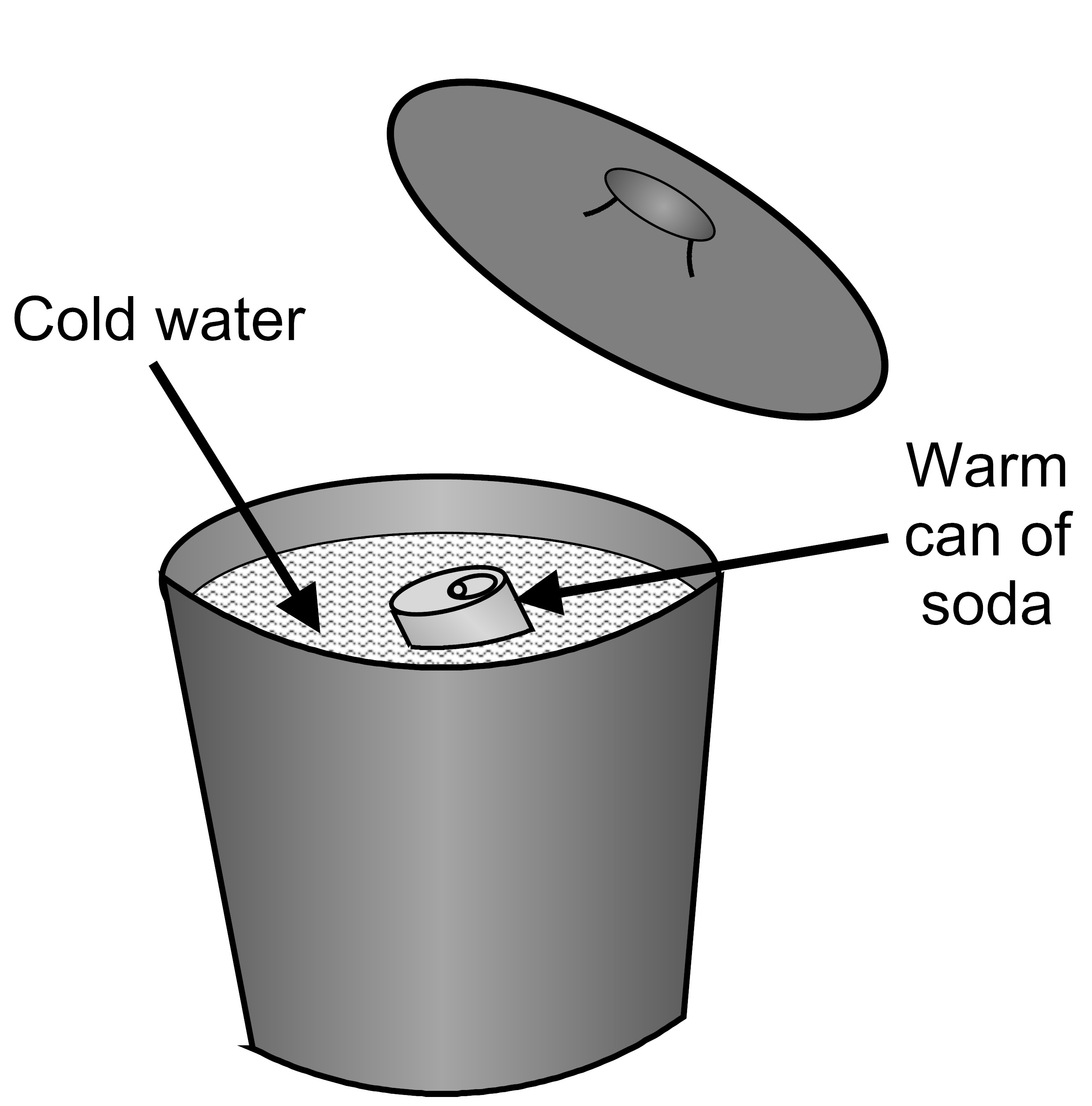
She puts the lid on the bucket. Which of the following describes the energy transfer between the water and the can of soda in the bucket?
- Thermal energy is transferred from the can of soda to the water so the can of soda gets cooler and the water stays the same temperature.
- Thermal energy is transferred from the can of soda to the water so the can of soda gets cooler and the water gets warmer.
- Coldness is transferred from the water to the can of soda so the can of soda gets cooler and the water stays the same temperature.
- Coldness is transferred from the water to the can of soda so the can of soda gets cooler and the water ice gets warmer.
- Distribution of Responses

- Students Responding Correctly
| Group | Correct | Total | Percent |
|---|---|---|---|
| Overall | 1672 | 4422 | 38% |
| Grades | |||
| 6–8 | 937 | 2812 | 33% |
| 9–12 | 730 | 1597 | 46% |
| Gender | |||
| Male | 885 | 2194 | 40% |
| Female | 762 | 2156 | 35% |
| Primary Language | |||
| English | 1512 | 3980 | 38% |
| Other | 127 | 341 | 37% |

